M2 macrophages induce ovarian cancer cell proliferation via a heparin binding epidermal growth factor/matrix metalloproteinase 9 intercellular feedback loop
- PMID: 27888810
- PMCID: PMC5349939
- DOI: 10.18632/oncotarget.13474
M2 macrophages induce ovarian cancer cell proliferation via a heparin binding epidermal growth factor/matrix metalloproteinase 9 intercellular feedback loop
Abstract
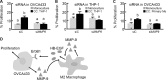
In ovarian cancer, a high ratio of anti-inflammatory M2 to pro-inflammatory M1 macrophages correlates with poor patient prognosis. The mechanisms driving poor tumor outcome as a result of the presence of M2 macrophages in the tumor microenvironment remain unclear and are challenging to study with current techniques. Therefore, in this study we utilized a micro-culture device previously developed by our lab to model concentrated paracrine signaling in order to address our hypothesis that interactions between M2 macrophages and ovarian cancer cells induce tumor cell proliferation. Using the micro-culture device, we determined that co-culture with M2-differentiated primary macrophages or THP-1 increased OVCA433 proliferation by 10-12%. This effect was eliminated with epidermal growth factor receptor (EGFR) or heparin-bound epidermal growth factor (HB-EGF) neutralizing antibodies and HBEGF expression in peripheral blood mononuclear cells from ovarian cancer patients was 9-fold higher than healthy individuals, suggesting a role for HB-EGF in tumor progression. However, addition of HB-EGF at levels secreted by macrophages or macrophage-conditioned media did not induce proliferation to the same extent, indicating a role for other factors in this process. Matrix metalloproteinase-9, MMP-9, which cleaves membrane-bound HB-EGF, was elevated in co-culture and its inhibition decreased proliferation. Utilizing inhibitors and siRNA against MMP9 in each population, we determined that macrophage-secreted MMP-9 released HB-EGF from macrophages, which increased MMP9 in OVCA433, resulting in a positive feedback loop to drive HB-EGF release and increase proliferation in co-culture. Identification of multi-cellular interactions such as this may provide insight into how to most effectively control ovarian cancer progression.
Keywords: bi-directional communication; co-culture; heparin binding epidermal growth factor; matrix metalloproteinase 9; paracrine signaling.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


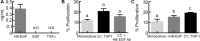
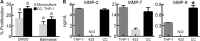
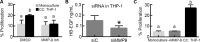

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

