NUP98 Fusion Proteins Interact with the NSL and MLL1 Complexes to Drive Leukemogenesis
- PMID: 27889185
- PMCID: PMC5501282
- DOI: 10.1016/j.ccell.2016.10.019
NUP98 Fusion Proteins Interact with the NSL and MLL1 Complexes to Drive Leukemogenesis
Abstract
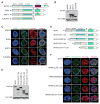
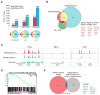
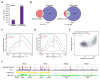
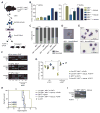
The nucleoporin 98 gene (NUP98) is fused to a variety of partner genes in multiple hematopoietic malignancies. Here, we demonstrate that NUP98 fusion proteins, including NUP98-HOXA9 (NHA9), NUP98-HOXD13 (NHD13), NUP98-NSD1, NUP98-PHF23, and NUP98-TOP1 physically interact with mixed lineage leukemia 1 (MLL1) and the non-specific lethal (NSL) histone-modifying complexes. Chromatin immunoprecipitation sequencing illustrates that NHA9 and MLL1 co-localize on chromatin and are found associated with Hox gene promoter regions. Furthermore, MLL1 is required for the proliferation of NHA9 cells in vitro and in vivo. Inactivation of MLL1 leads to decreased expression of genes bound by NHA9 and MLL1 and reverses a gene expression signature found in NUP98-rearranged human leukemias. Our data reveal a molecular dependency on MLL1 function in NUP98-fusion-driven leukemogenesis.
Keywords: Hox gene; NUP98 fusion; leukemia; mixed lineage leukemia 1; non-specific lethal histone-modifying complexes.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

