Acantholytic squamous cell carcinoma is usually associated with hair follicles, not acantholytic actinic keratosis, and is not "high risk": Diagnosis, management, and clinical outcomes in a series of 115 cases
- PMID: 27889291
- PMCID: PMC5319832
- DOI: 10.1016/j.jaad.2016.09.024
Acantholytic squamous cell carcinoma is usually associated with hair follicles, not acantholytic actinic keratosis, and is not "high risk": Diagnosis, management, and clinical outcomes in a series of 115 cases
Abstract
Background: Acantholytic squamous cell carcinoma (aSCC) is regarded as a high-risk variant of cutaneous squamous cell carcinoma (SCC). Acantholytic actinic keratosis (aAK) has been regarded as a precursor risk factor for aSCC. However, supporting evidence is limited.
Objective: We sought to document clinical features, histologic features, management, and outcomes in a series of aSCC cases.
Methods: Definitions of aSCC, aAK, and aSCC arising in association with aAK were applied to a consecutive series of aSCC cases. Clinical characteristics and outcomes were obtained from electronic medical records.
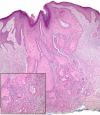
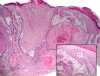
Results: Of 115 aSCC cases (103 patients, mean age 71.8 years), actinic keratosis was present in 23% (27/115) but only 7.8% (9/115) exhibited associated aAK. Ten cases (10/115, 9%) fulfilled strict histologic criteria for follicular SCC as previously defined, but 50 of 115 (43%) of our aSCC cases exhibited predominant involvement of follicular epithelium rather than epidermis. Clinical outcome (median follow-up, 36 months) was available in 106 of 115 (92%). One patient experienced regional extension (parotid), and 1 patient experienced a local recurrence (nose). No disease-related metastases or deaths were documented.
Limitations: This was a single-institution retrospective study from the United States.
Conclusions: The presence of acantholysis in cutaneous SCC does not specifically confer aggressive behavior, a finding that may inform clinical practice guidelines.
Keywords: acantholysis; acantholytic actinic keratosis; cutaneous oncology; dermatopathology; follicular squamous cell carcinoma; nonmelanoma skin cancer; outcomes; prognosis; squamous cell carcinoma.
Copyright © 2016 American Academy of Dermatology, Inc. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Cassarino DS, Derienzo DP, Barr RJ. Cutaneous squamous cell carcinoma: a comprehensive clinicopathologic classification. Part one. J Cutan Pathol. 2006;33(3):191–206. - PubMed
-
- Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. J Am Acad Dermatol. 2013;68(6):957–966. - PubMed
-
- Navarrete-Dechent C, Veness MJ, Droppelmann N, Uribe P. High-risk cutaneous squamous cell carcinoma and the emerging role of sentinel lymph node biopsy: a literature review. J Am Acad Dermatol. 2015;73(1):127–137. - PubMed
-
- Lever WF. Adenocanthoma of sweat glands; carcinoma of sweat glands with glandular and epidermal elements: report of four cases. Arch Dermatol Syphilol. 1947;56(2):157–171. - PubMed
-
- LeBoit PE, Burg G, Weedon D, Sarasin A. Pathology and genetics of skin tumors. 3rd ed. IARC Press; Lyon (France): 2005. World Health Organization classification of tumors.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

