Adverse events and treatment discontinuations of antimuscarinics for the treatment of overactive bladder in older adults: A systematic review and meta-analysis
- PMID: 27889591
- PMCID: PMC5516911
- DOI: 10.1016/j.archger.2016.11.006
Adverse events and treatment discontinuations of antimuscarinics for the treatment of overactive bladder in older adults: A systematic review and meta-analysis
Abstract
Introduction: Antimuscarinics should be used with caution in older adults with overactive bladder (OAB) due to anticholinergic adverse events (AEs). Systematic reviews and meta-analyses (SRMAs) have analyzed safety-related outcomes but have not specified risk in the elderly, the population at highest risk for AEs. The aim of this review is to explore and evaluate AEs and treatment discontinuations in adults 65 or older taking antimuscarinics for OAB.
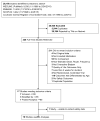
Methods: Keywords were searched in MEDLINE, EMBASE, SCOPUS, and Cochrane Central Register for Controlled Trials. Randomized controlled trials (RCTs) along with sub-analyses and pooled analyses that compared antimuscarinics to placebo or another antimuscarinic were performed in February 2015. Studies assessing AEs or treatment discontinuations in a population of adults 65 or older were included. The Jadad Criteria and McHarm Tool were used to assess the quality of the trials.
Results: A total of 16 studies met the inclusion criteria. Eighty AEs and 27 reasons for treatment discontinuation were described in the included studies and further explored. Anticholinergic AEs were more common in antimuscarinics compared to placebo. Incidence of dizziness, dyspepsia, and urinary retention with fesoterodine, headache with darifenacin, and urinary tract infections with solifenacin were significantly higher compared to placebo. Treatment discontinuation due to AEs and dry mouth were higher in the antimuscarinics when compared to placebo in older adults.
Conclusions: Treatment for overactive bladder using antimuscarinics in adults aged 65 or older resulted in significant increases in risk for several AEs compared to placebo including anticholinergic and non-anticholinergic AEs.
Keywords: Adverse events; Antimuscarinics; Elderly; Meta-analysis; Overactive bladder; Systematic review.
Copyright © 2016 Elsevier Ireland Ltd. All rights reserved.
Comment in
-
Re: Adverse Events and Treatment Discontinuations of Antimuscarinics for the Treatment of Overactive Bladder in Older Adults: A Systematic Review and Meta-Analysis.J Urol. 2018 Jan;199(1):10-11. doi: 10.1016/j.juro.2017.09.127. Epub 2017 Oct 10. J Urol. 2018. PMID: 29310152 No abstract available.
References
-
- National Cancer Institute Common Terminology Criteria for Adverse Events v3.0 (CTCAE) 2009.
-
- Appell RA, Sand P, Dmochowski R, Anderson R, Zinner N, Lama D, Roach M, Miklos J, Saltzstein D, Boone T, Staskin DR, Albrecht D. Prospective randomized controlled trial of extended-release oxybutynin chloride and tolterodine tartrate in the treatment of overactive bladder: results of the OBJECT Study. Mayo Clinic proceedings. 2001;76:358–363. - PubMed
-
- Benner JS, Nichol MB, Rovner ES, Jumadilova Z, Alvir J, Hussein M, Fanning K, Trocio JN, Brubaker L. Patient-reported reasons for discontinuing overactive bladder medication. BJU international. 2010;105:1276–1282. - PubMed
-
- Cardozo L, Lisec M, Millard R, van Vierssen Trip O, Kuzmin I, Drogendijk TE, Huang M, Ridder AM. Randomized, double-blind placebo controlled trial of the once daily antimuscarinic agent solifenacin succinate in patients with overactive bladder. The Journal of urology. 2004;172:1919–1924. - PubMed
-
- Chapple C, DuBeau C, Ebinger U, Rekeda L, Viegas A. Darifenacin treatment of patients >or= 65 years with overactive bladder: results of a randomized, controlled, 12-week trial. Current medical research and opinion. 2007a;23:2347–2358. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous