Mirvetuximab Soravtansine (IMGN853), a Folate Receptor Alpha-Targeting Antibody-Drug Conjugate, Potentiates the Activity of Standard of Care Therapeutics in Ovarian Cancer Models
- PMID: 27889646
- PMCID: PMC5126132
- DOI: 10.1016/j.neo.2016.11.002
Mirvetuximab Soravtansine (IMGN853), a Folate Receptor Alpha-Targeting Antibody-Drug Conjugate, Potentiates the Activity of Standard of Care Therapeutics in Ovarian Cancer Models
Abstract
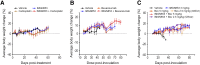
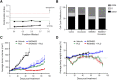
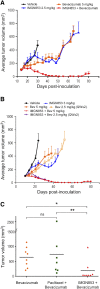
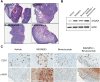
Elevated folate receptor alpha (FRα) expression is characteristic of epithelial ovarian cancer (EOC), thus establishing this receptor as a candidate target for the development of novel therapeutics to treat this disease. Mirvetuximab soravtansine (IMGN853) is an antibody-drug conjugate (ADC) that targets FRα for tumor-directed delivery of the maytansinoid DM4, a potent agent that induces mitotic arrest by suppressing microtubule dynamics. Here, combinations of IMGN853 with approved therapeutics were evaluated in preclinical models of EOC. Combinations of IMGN853 with carboplatin or doxorubicin resulted in synergistic antiproliferative effects in the IGROV-1 ovarian cancer cell line in vitro. IMGN853 potentiated the cytotoxic activity of carboplatin via growth arrest and augmented DNA damage; cell cycle perturbations were also observed in cells treated with the IMGN853/doxorubicin combination. These benefits translated into improved antitumor activity in patient-derived xenograft models in vivo in both the platinum-sensitive (IMGN853/carboplatin) and platinum-resistant (IMGN853/pegylated liposomal doxorubicin) settings. IMGN853 co-treatment also improved the in vivo efficacy of bevacizumab in platinum-resistant EOC models, with combination regimens causing significant regressions and complete responses in the majority of tumor-bearing mice. Histological analysis of OV-90 ovarian xenograft tumors revealed that concurrent administration of IMGN853 and bevacizumab caused rapid disruption of tumor microvasculature and extensive necrosis, underscoring the superior bioactivity profile of the combination regimen. Overall, these demonstrations of combinatorial benefit conferred by the addition of the first FRα-targeting ADC to established therapies provide a compelling framework for the potential application of IMGN853 in the treatment of patients with advanced ovarian cancer.
Copyright © 2016 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Siegel R., Ma J., Zou Z., Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. - PubMed
-
- Society AC . American Cancer Society; 2016. Cancer facts & figures.
-
- Lacey J.V., Sherman M.E. In: Ovarian neoplasia. Robboy S.L., Mutter G.L., Prat J., editors. vol. 2. Churchill Livingstone Elsevier; Oxford: 2009. p. 601.
-
- Liu J., Matulonis U.A. New strategies in ovarian cancer: translating the molecular complexity of ovarian cancer into treatment advances. Clin Cancer Res. 2014;20:5150–5156. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

