Interprofessional Medication Management in Patients With Multiple Morbidities
- PMID: 27890050
- PMCID: PMC5159681
- DOI: 10.3238/arztebl.2016.0741
Interprofessional Medication Management in Patients With Multiple Morbidities
Abstract
Background: Medication reviews and medication management are being used more and more around the world to improve medication safety. Both of these tools were originally conceived as pharmaceutical care activities and have recently been developed into interdisciplinary approaches. We studied the efficacy of interprofessional medication management for multimorbid patients that takes their medical conditions, but also their general living situation into account.
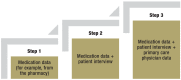
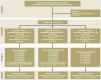
Methods: A comprehensive medication management was performed, which involved the collection of information on the drugs each patient took, the way they were stored, the patient's drug intake and handling, and any problems that arose with pharmacotherapy. The interventional approach was evaluated over a period of 15 months in a cluster-randomized controlled trial with a stepped wedge design. The primary endpoint was the quality of pharmacotherapy, as assessed with the Medication Appropriateness Index (MAI). A mixed model was used to analyze efficacy.
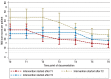
Results: 162 patients were enrolled in the study; 142 were included in the intention-to-treat analysis (53.3% women, mean age 76.8 ± 6.3 years). The mean total MAI score decreased significantly (p ≤ 0.001) from the control phase (29.21, 95% CI [26.09; 32.33]) to the intervention phase (22.27 [19.00; 25.54]), with an effect strength (Cohen's d) of -0.24 [-0.36; -0.13]. The number of drug-related problems declined as well.
Conclusion: In this study, interprofessional collaboration increased medication safety. Working across disciplinary boundaries allowed for a decrease in drugrelated problems and brought up aspects outside the purview of the primary care physician.
Figures
Comment in
-
Medication Safety-Models of Interprofessional Collaboration.Dtsch Arztebl Int. 2016 Nov 4;113(43):739-740. doi: 10.3238/arztebl.2016.0739. Dtsch Arztebl Int. 2016. PMID: 27890049 Free PMC article. No abstract available.
-
Avoiding Errors in Chemotherapy.Dtsch Arztebl Int. 2017 Mar 31;114(13):224. doi: 10.3238/arztebl.2017.0224a. Dtsch Arztebl Int. 2017. PMID: 28434440 Free PMC article. No abstract available.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

