Bupropion sustained release for pregnant smokers: a randomized, placebo-controlled trial
- PMID: 27890648
- PMCID: PMC5376363
- DOI: 10.1016/j.ajog.2016.11.1036
Bupropion sustained release for pregnant smokers: a randomized, placebo-controlled trial
Abstract
Background: Bupropion is used to treat depression during pregnancy. However, its usefulness as a smoking cessation aid for pregnant women is not fully known.
Objective: The objective of the study was to evaluate the preliminary efficacy of bupropion sustained release for smoking cessation during pregnancy.
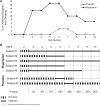
Study design: We conducted a randomized, prospective, double-blind, placebo-controlled, pilot trial. Pregnant women who smoked daily received individualized behavior counseling and were randomly assigned to a 12 week, twice-a-day treatment with 150 mg bupropion sustained release or placebo. The primary study objectives were to determine whether bupropion sustained release reduces nicotine withdrawal symptoms on the quit date and during the treatment period compared with placebo and whether it increases 7 day point prevalence abstinence at the end of the treatment period and at the end of pregnancy.
Results: Subjects in the bupropion (n = 30) and placebo (n = 35) groups were comparable in age, smoking history, number of daily smoked cigarettes, and nicotine dependence. After controlling for maternal age and race, bupropion sustained release reduced cigarette cravings (1.5 ± 1.1 vs 2.1 ± 1.2, P = .02) and total nicotine withdrawal symptoms (3.8 ± 4.3 vs 5.4 ± 5.1, P = .028) during the treatment period. Administration of bupropion sustained release reduced tobacco exposure, as determined by levels of carbon monoxide in exhaled air (7.4 ± 6.4 vs 9.1 ± 5.8, P = .053) and concentrations of cotinine in urine (348 ± 384 ng/mL vs 831 ± 727 ng/mL, P = .007) and increased overall abstinence rates during treatment (19% vs 2%, P = .003). However, there was no significant difference in 7 day point prevalence abstinence rates between the 2 groups at the end of medication treatment (17% vs 3%, P = .087) and at the end of pregnancy (10% vs 3%, P = .328).
Conclusion: Individual smoking cessation counseling along with the twice-daily use of 150 mg bupropion sustained release increased smoking cessation rates and reduced cravings and total nicotine withdrawal symptoms during the treatment period. However, there was no significant difference in abstinence rates between groups at the end of medication treatment and at the end of pregnancy, likely because of the small sample size. A larger study is needed to confirm these findings and to examine the potential benefit/ risk ratio of bupropion sustained release for smoking cessation during pregnancy.
Keywords: bupropion sustained release; pregnancy; smoking cessation.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures

References
-
- Patnode CP, Henderson JT, Thompson JH, Senger CA, Fortmann SP, Whitlock EP. Behavioral Counseling and Pharmacotherapy Interventions for Tobacco Cessation in Adults, Including Pregnant Women: A Review of Reviews for the U.S. Preventive Services Task Force. Agency for Healthcare Research and Quality; Rockville, MD: Sep, 2015. Evidence Synthesis No. 134. AHRQ Publication No. 14-05200-EF-1. (Evidence Syntheses, No. 134. - PubMed
-
- Fiore MC, Jaén CR, Baker TB, et al. Treating tobacco use and dependence: 2008 update U.S. Public Health Service Clinical Practice Guideline executive summary. Respir Care. 2008;53(9):1217–22. PubMed PMID: 18807274. - PubMed
-
- Lumley J, Oliver SS, Chamberlain C, Oakley L. Interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev. 2004;(4):CD001055. DOI: 10.1002/14651858.CD001055.pub2. PubMed PMID: 15495004. - PubMed
-
- Raupach T, van Schayck CP. Pharmacotherapy for smoking cessation: current advances and research topics. CNS Drugs. 2011;25(5):371–82. - PubMed
-
- Chan B, Einarson A, Koren G. Effectiveness of bupropion for smoking cessation during pregnancy. J Addict Dis. 2005;24(2):19–23. DOI: 10.1300/J069v24n02_02. PubMed PMID: 15784520. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

