Cyclosporin derivatives inhibit hepatitis B virus entry without interfering with NTCP transporter activity
- PMID: 27890789
- PMCID: PMC7172969
- DOI: 10.1016/j.jhep.2016.11.009
Cyclosporin derivatives inhibit hepatitis B virus entry without interfering with NTCP transporter activity
Abstract
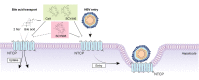
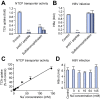
Background & aims: The sodium taurocholate co-transporting polypeptide (NTCP) is the main target of most hepatitis B virus (HBV) specific entry inhibitors. Unfortunately, these agents also block NTCP transport of bile acids into hepatocytes, and thus have the potential to cause adverse effects. We aimed to identify small molecules that inhibit HBV entry while maintaining NTCP transporter function.
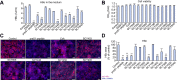
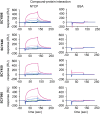
Methods: We characterized a series of cyclosporine (CsA) derivatives for their anti-HBV activity and NTCP binding specificity using HepG2 cells overexpressing NTCP and primary human hepatocytes. The four most potent derivatives were tested for their capacity to prevent HBV entry, but maintain NTCP transporter function. Their antiviral activity against different HBV genotypes was analysed.
Results: We identified several CsA derivatives that inhibited HBV infection with a sub-micromolar IC50. Among them, SCY446 and SCY450 showed low activity against calcineurin (CN) and cyclophilins (CyPs), two major CsA cellular targets. This suggested that instead, these compounds interacted directly with NTCP to inhibit viral attachment to host cells, and have no immunosuppressive function. Importantly, we found that SCY450 and SCY995 did not impair the NTCP-dependent uptake of bile acids, and inhibited multiple HBV genotypes including a clinically relevant nucleoside analog-resistant HBV isolate.
Conclusions: This is the first example of small molecule selective inhibition of HBV entry with no decrease in NTCP transporter activity. It suggests that the anti-HBV activity can be functionally separated from bile acid transport. These broadly active anti-HBV molecules are potential candidates for developing new drugs with fewer adverse effects.
Lay summary: In this study, we identified new compounds that selectively inhibited hepatitis B virus (HBV) entry, and did not impair bile acid uptake. Our evidence offers a new strategy for developing anti-HBV drugs with fewer side effects.
Keywords: Antiviral; Bile acids and salts; Cyclophilins; Cyclosporine; HBV; Hepatitis B virus; Hepatitis D virus; Infection; Membrane transport proteins; NTCP; PreS1; Replication.
Copyright © 2016 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Figures


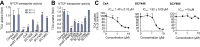

Comment in
-
Advancing hepatitis B virus entry inhibitors.J Hepatol. 2017 Apr;66(4):677-679. doi: 10.1016/j.jhep.2016.11.028. Epub 2016 Dec 10. J Hepatol. 2017. PMID: 27965159 Free PMC article. No abstract available.
References
-
- Ott J.J., Stevens G.A., Groeger J., Wiersma S.T. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. 2012;30:2212–2219. - PubMed
-
- Zeisel M.B., Lucifora J., Mason W.S., Sureau C., Beck J., Levrero M. Towards an HBV cure: state-of-the-art and unresolved questions-report of the ANRS workshop on HBV cure. Gut. 2015;64:1314–1326. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

