Validation of biomarkers to predict response to immunotherapy in cancer: Volume II - clinical validation and regulatory considerations
- PMID: 27891226
- PMCID: PMC5109653
- DOI: 10.1186/s40425-016-0179-0
Validation of biomarkers to predict response to immunotherapy in cancer: Volume II - clinical validation and regulatory considerations
Abstract
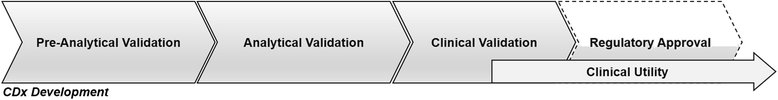
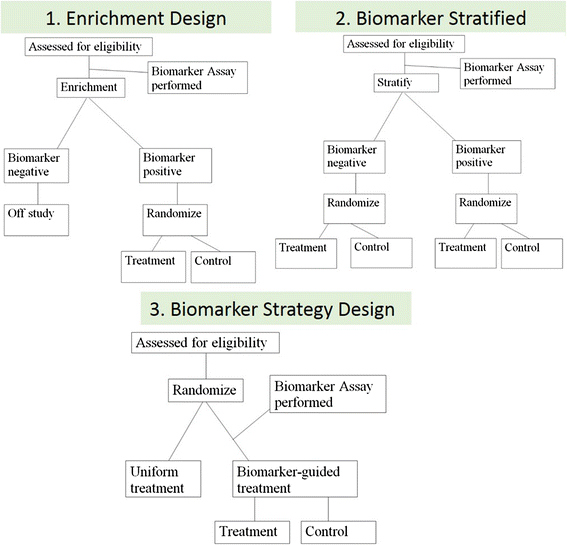
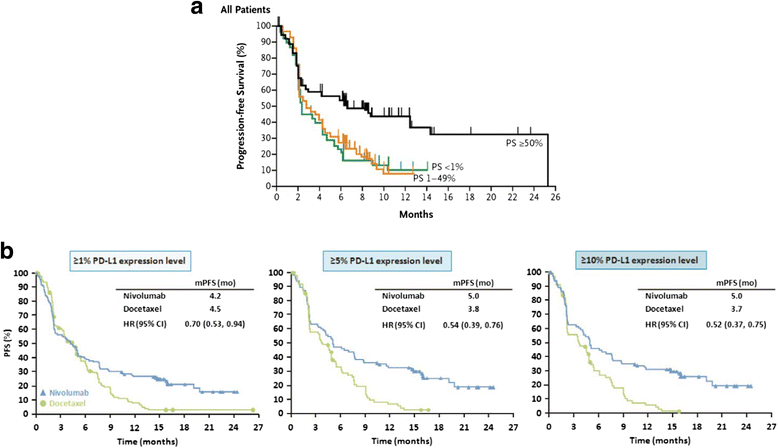
There is growing recognition that immunotherapy is likely to significantly improve health outcomes for cancer patients in the coming years. Currently, while a subset of patients experience substantial clinical benefit in response to different immunotherapeutic approaches, the majority of patients do not but are still exposed to the significant drug toxicities. Therefore, a growing need for the development and clinical use of predictive biomarkers exists in the field of cancer immunotherapy. Predictive cancer biomarkers can be used to identify the patients who are or who are not likely to derive benefit from specific therapeutic approaches. In order to be applicable in a clinical setting, predictive biomarkers must be carefully shepherded through a step-wise, highly regulated developmental process. Volume I of this two-volume document focused on the pre-analytical and analytical phases of the biomarker development process, by providing background, examples and "good practice" recommendations. In the current Volume II, the focus is on the clinical validation, validation of clinical utility and regulatory considerations for biomarker development. Together, this two volume series is meant to provide guidance on the entire biomarker development process, with a particular focus on the unique aspects of developing immune-based biomarkers. Specifically, knowledge about the challenges to clinical validation of predictive biomarkers, which has been gained from numerous successes and failures in other contexts, will be reviewed together with statistical methodological issues related to bias and overfitting. The different trial designs used for the clinical validation of biomarkers will also be discussed, as the selection of clinical metrics and endpoints becomes critical to establish the clinical utility of the biomarker during the clinical validation phase of the biomarker development. Finally, the regulatory aspects of submission of biomarker assays to the U.S. Food and Drug Administration as well as regulatory considerations in the European Union will be covered.
Keywords: Assay; Biomarker; Cancer; Immunotherapy; Regulatory; Validation.
Figures


References
-
- Institute of Medicine . The national academies collection: reports funded by national institutes of health, transforming clinical research in the United States: challenges and opportunities: workshop summary. Washington DC: National Academies Press; 2010. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
