The effectiveness of regional cooling for paclitaxel-induced peripheral neuropathy
- PMID: 27891244
- PMCID: PMC5111235
- DOI: 10.1186/s40780-016-0067-2
The effectiveness of regional cooling for paclitaxel-induced peripheral neuropathy
Abstract
Background: There are currently no promising therapies available to treat or prevent peripheral neuropathy (PN) induced by anticancer drugs in a cumulative dose-dependent manner. In this study, we investigated the efficacy of regional cooling of hands and feet in preventing paclitaxel (PTX)-induced PN.
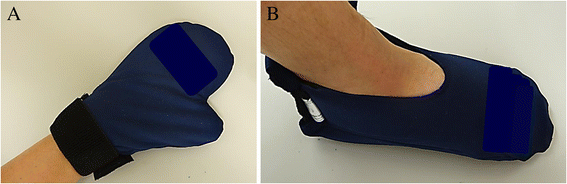
Methods: Patients with gynecologic cancer who received a tri-weekly cycle of chemotherapy including PTX at doses of 150-175 mg/m2 were included in this study. Regional cooling was performed by covering patient hands and feet with cold insulators during PTX administration (regional cooling group). The primary end-point was ≥grade 2 PN evaluated by the Common Terminology Criteria for Adverse Events (CTCAE) v4.0. The secondary end-points were the frequency of PN therapeutic drug use, PTX dose reduction due to PN, and adverse events due to regional cooling. The efficacy of regional cooling was compared with data retrospectively extracted from the medical records of patients who did not receive regional cooling (control group). All end-points were evaluated for up to six cycles.
Results: There were 40 and 142 patients in the regional cooling and control groups, respectively. As a primary end-point, incidences of ≥grade 2 PN in the fourth to sixth cycles were significantly lower than that in the cooling group (5.0-9.1 % vs. 19.8-31.6 %, p < 0.05 after the fourth cycle and p < 0.01 after the fifth cycle). Among secondary end-points, neither the use of PN therapeutic drugs nor the PTX dose reduction due to PN were significantly lower in the cooling group than in the control group (27.5 vs. 36.6 %, p = 0.378 and 5.0 vs. 3.5 %, p = 0.645, respectively). There were no serious regional cooling-associated adverse events such as frostbite.
Conclusions: Regional cooling of hands and feet during PTX administration might have good effectiveness and tolerability, suggesting this approach as a potentially effective supportive care to prevent PTX-induced PN.
Trial registration: The trial approval number in the institution; H25-26. Registered 5 June 2014.
Keywords: Chemotherapy; Gynecologic cancer; Paclitaxel; Peripheral neuropathy; Regional cooling.
Figures


References
-
- Arakawa K, Torigoe K, Kuzumaki N, Suzuki T, Narita M. Chemothrapy-induced peripheral neuropathy: clinical symptoms, managements and mechanism. Jpn J Pharm Palliat Care Sci. 2011;4:1–13.
-
- Katsumata N, Yasuda M, Takahashi F, Isonishi S, Jobo T, Aoki D, et al. Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a phase 3, open-label, randomised controlled trial. Lancet. 2009;374:1331–1338. doi: 10.1016/S0140-6736(09)61157-0. - DOI - PubMed
-
- Piccart J, Bertelsen K, James K, Cassidy J, Mangioni C, Simonsen E, et al. Randomized intergroup trial of cisplatin-paclitaxel versus cisplatin-cyclophosphamide in women with advanced epithelial ovarian cancer: three-year results. J Natl Cancer Inst. 2000;92:699–708. doi: 10.1093/jnci/92.9.699. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

