Diacylglycerol Kinases in T Cell Tolerance and Effector Function
- PMID: 27891502
- PMCID: PMC5103287
- DOI: 10.3389/fcell.2016.00130
Diacylglycerol Kinases in T Cell Tolerance and Effector Function
Abstract
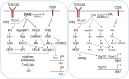
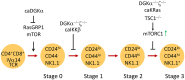
Diacylglycerol kinases (DGKs) are a family of enzymes that regulate the relative levels of diacylglycerol (DAG) and phosphatidic acid (PA) in cells by phosphorylating DAG to produce PA. Both DAG and PA are important second messengers cascading T cell receptor (TCR) signal by recruiting multiple effector molecules, such as RasGRP1, PKCθ, and mTOR. Studies have revealed important physiological functions of DGKs in the regulation of receptor signaling and the development and activation of immune cells. In this review, we will focus on recent progresses in our understanding of two DGK isoforms, α and ζ, in CD8 T effector and memory cell differentiation, regulatory T cell development and function, and invariant NKT cell development and effector lineage differentiation.
Keywords: diacylglycerol kinase; invariant NKT cells; regulatory T cells.
Figures
References
-
- Alonso R., Mazzeo C., Rodriguez M. C., Marsh M., Fraile-Ramos A., Calvo V., et al. (2011). Diacylglycerol kinase α regulates the formation and polarisation of mature multivesicular bodies involved in the secretion of Fas ligand-containing exosomes in T lymphocytes. Cell Death Differ. 18, 1161–1173. 10.1038/cdd.2010.184 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

