Association between antibiotic use among pregnant women with urinary tract infections in the first trimester and birth defects, National Birth Defects Prevention Study 1997 to 2011
- PMID: 27891788
- PMCID: PMC5225492
- DOI: 10.1002/bdra.23570
Association between antibiotic use among pregnant women with urinary tract infections in the first trimester and birth defects, National Birth Defects Prevention Study 1997 to 2011
Abstract
Background: Previous studies noted associations between birth defects and some antibiotics (e.g., nitrofurantoin, sulfonamides) but not others (e.g., penicillins). It is unclear if previous findings were due to antibiotic use, infections, or chance. To control for potential confounding by indication, we examined associations between antibiotic use and birth defects, among women reporting urinary tract infections (UTIs).
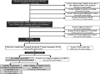
Methods: The National Birth Defects Prevention Study is a multi-site, population-based case-control study. Case infants/fetuses have any of over 30 major birth defects and controls are live-born infants without major birth defects. We analyzed pregnancies from 1997 to 2011 to estimate the association between maternally reported periconceptional (month before conception through the third month of pregnancy) use of nitrofurantoin, trimethoprim-sulfamethoxazole, or cephalosporins and specific birth defects, among women with periconceptional UTIs. Women with periconceptional UTIs who reported penicillin use served as the comparator.
Results: Periconceptional UTIs were reported by 7.8% (2029/26,068) of case and 6.7% (686/10,198) of control mothers. Most (68.2% of case, 66.6% of control mothers) also reported antibiotic use. Among 608 case and 231 control mothers reporting at least one periconceptional UTI and certain antibiotic use, compared with penicillin, nitrofurantoin use was associated with oral clefts in the offspring (adjusted odds ratio, 1.97 [95% confidence interval, 1.10-3.53]), trimethoprim-sulfamethoxazole use with esophageal atresia (5.31 [1.39-20.24]) and diaphragmatic hernia (5.09 [1.20-21.69]), and cephalosporin use with anorectal atresia/stenosis (5.01 [1.34-18.76]).
Conclusion: Periconceptional exposure to some antibiotics might increase the risk for certain birth defects. However, because individual birth defects are rare, absolute risks should drive treatment decisions.Birth Defects Research (Part A) 106:940-949, 2016.© 2016 Wiley Periodicals, Inc.
Keywords: antibiotic; birth defects; cephalosporin; nitrofurantoin; penicillin; trimethoprim-sulfamethoxazole; urinary tract infection.
© 2016 Wiley Periodicals, Inc.
Conflict of interest statement
None of the authors have any conflicts of interest to disclose.
Figures

References
-
- American College of Obstetricians and Gynecologists Committee on Obstetric Practice. Committee Opinion No. 494: sulfonamides, nitrofurantoin, and risk of birth defects. Obstet Gynecol. 2011;117:1484–1485. - PubMed
-
- Bhutta ZA, Yusuf K. Early-onset neonatal sepsis in Pakistan: a case control study of risk factors in a birth cohort. Am J Perinatol. 1997;14:577–581. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

