Liver-specific ATP-citrate lyase inhibition by bempedoic acid decreases LDL-C and attenuates atherosclerosis
- PMID: 27892461
- PMCID: PMC5133702
- DOI: 10.1038/ncomms13457
Liver-specific ATP-citrate lyase inhibition by bempedoic acid decreases LDL-C and attenuates atherosclerosis
Abstract
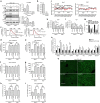
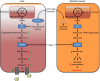
Despite widespread use of statins to reduce low-density lipoprotein cholesterol (LDL-C) and associated atherosclerotic cardiovascular risk, many patients do not achieve sufficient LDL-C lowering due to muscle-related side effects, indicating novel treatment strategies are required. Bempedoic acid (ETC-1002) is a small molecule intended to lower LDL-C in hypercholesterolemic patients, and has been previously shown to modulate both ATP-citrate lyase (ACL) and AMP-activated protein kinase (AMPK) activity in rodents. However, its mechanism for LDL-C lowering, efficacy in models of atherosclerosis and relevance in humans are unknown. Here we show that ETC-1002 is a prodrug that requires activation by very long-chain acyl-CoA synthetase-1 (ACSVL1) to modulate both targets, and that inhibition of ACL leads to LDL receptor upregulation, decreased LDL-C and attenuation of atherosclerosis, independently of AMPK. Furthermore, we demonstrate that the absence of ACSVL1 in skeletal muscle provides a mechanistic basis for ETC-1002 to potentially avoid the myotoxicity associated with statin therapy.
Conflict of interest statement
S.L.P., R.S.N., C.M.B., S.F., P.H.E.G., G.R.S. and N.D.L. received compensation from Esperion Therapeutics, Inc. The remaining authors declare no competing financial interests.
Figures




References
-
- Mendis S. The contribution of the Framingham Heart Study to the prevention of cardiovascular disease: a global perspective. Prog. Cardiovasc. Dis. 53, 10–14 (2010). - PubMed
-
- Ference B. A. & Mahajan N. The role of early LDL lowering to prevent the onset of atherosclerotic disease. Curr. Atheroscler. Rep. 15, 312 (2013). - PubMed
-
- Stone N. J. et al.. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 129, S1–45 (2014). - PubMed
-
- Taylor F. C., Huffman M. & Ebrahim S. Statin therapy for primary prevention of cardiovascular disease. JAMA 310, 2451–2452 (2013). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

