Autophagy is required for PDAC glutamine metabolism
- PMID: 27892481
- PMCID: PMC5124864
- DOI: 10.1038/srep37594
Autophagy is required for PDAC glutamine metabolism
Abstract
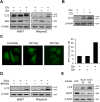
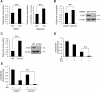
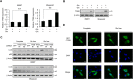
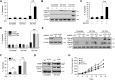
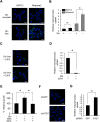
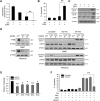
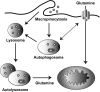
Macroautophagy (autophagy) is believed to maintain energy homeostasis by degrading unnecessary cellular components and molecules. Its implication in regulating cancer metabolism recently started to be uncovered. However, the precise roles of autophagy in cancer metabolism are still unclear. Here, we show that autophagy plays a critical role in glutamine metabolism, which is required for tumor survival. Pancreatic ductal adenocarcinoma (PDAC) cells require both autophagy and typical glutamine transporters to maintain intracellular glutamine levels. Glutamine deprivation, but not that of glucose, led to the activation of macropinocytosis-associated autophagy through TFEB induction and translocation into the nucleus. In contrast, glutamine uptake increased as a compensatory response to decreased intracellular glutamine levels upon autophagy inhibition. Moreover, autophagy inhibition and glutamine deprivation did not induce cell death, while glutamine deprivation dramatically activated apoptotic cell death upon autophagy inhibition. Interestingly, the addition of α-ketoglutarate significantly rescued the apoptotic cell death caused by the combination of the inhibition of autophagy with glutamine deprivation. Our data suggest that macropinocytosis-associated autophagy is a critical process providing glutamine for anaplerosis of the TCA cycle in PDAC. Thus, targeting both autophagy and glutamine metabolism to completely block glutamine supply may provide new therapeutic approaches to treat refractory tumors.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

