Dendritic cell function and pathogen-specific T cell immunity are inhibited in mice administered levonorgestrel prior to intranasal Chlamydia trachomatis infection
- PMID: 27892938
- PMCID: PMC5125275
- DOI: 10.1038/srep37723
Dendritic cell function and pathogen-specific T cell immunity are inhibited in mice administered levonorgestrel prior to intranasal Chlamydia trachomatis infection
Abstract
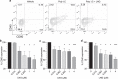
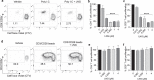
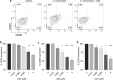
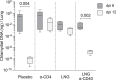
The growing popularity of levonorgestrel (LNG)-releasing intra-uterine systems for long-acting reversible contraception provides strong impetus to define immunomodulatory properties of this exogenous progestin. In initial in vitro studies herein, we found LNG significantly impaired activation of human dendritic cell (DCs) and their capacity to promote allogeneic T cell proliferation. In follow-up studies in a murine model of intranasal Chlamydia trachomatis infection, we analogously found that LNG treatment prior to infection dramatically reduced CD40 expression in DCs isolated from draining lymph nodes at 2 days post infection (dpi). At 12 dpi, we also detected significantly fewer CD4+ and CD8+ T cells in the lungs of LNG-treated mice. This inhibition of DC activation and T cell expansion in LNG-treated mice also delayed chlamydial clearance and the resolution of pulmonary inflammation. Conversely, administering agonist anti-CD40 monoclonal antibody to LNG-treated mice at 1 dpi restored lung T cell numbers and chlamydial burden at 12 dpi to levels seen in infected controls. Together, these studies reveal that LNG suppresses DC activation and function, and inhibits formation of pathogen-specific T cell immunity. They also highlight the need for studies that define in vivo effects of LNG use on human host response to microbial pathogens.
Figures


References
-
- Cleland J. Contraception in historical and global perspective. Best Pract. Res. Clin. Obstet. Gynaecol. 23, 165–176 (2009). - PubMed
-
- Clifton D. & Kaneda T. Family Planning Worldwide 2013 Data Sheet. Population Reference Bureau (date of access: 30/06/2016). http://www.prb.org/Publications/Datasheets/2013/family-planning-worldwid...(2013)
-
- Bayer Healthcare Pharmaceuticals. Skyla package insert. Wayne, NJ: Bayer Healthcare Pharmaceuticals (2016).
-
- Bayer Healthcare Pharmaceuticals. Mirena package insert. Wayne, NJ: Bayer Healthcare Pharmaceuticals (2016).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

