Potential of Small Molecule-Mediated Reprogramming of Rod Photoreceptors to Treat Retinitis Pigmentosa
- PMID: 27893103
- PMCID: PMC5134355
- DOI: 10.1167/iovs.16-20177
Potential of Small Molecule-Mediated Reprogramming of Rod Photoreceptors to Treat Retinitis Pigmentosa
Abstract
Purpose: Mutations in rod photoreceptor genes can cause retinitis pigmentosa (RP). Rod gene expression is regulated by the nuclear hormone receptor, Nr2e3. Genetic deletion of Nr2e3 reprograms rods into cells that resemble cone photoreceptors, and might therefore prevent their death from some forms of RP. There are no identified ligands for Nr2e3; however, reverse agonists might mimic the genetic rescue effect and may be therapeutically useful for the treatment of RP.
Methods: We screened for small molecule modulators of Nr2e3 using primary retinal cell cultures and characterized the most potent, which we have named photoregulin1 (PR1), in vitro and in vivo. We also tested the ability of PR1 to slow the progression of photoreceptor degeneration in two common mouse models of autosomal dominant RP, the RhoP23H and the Pde6brd1 mutations.
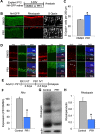
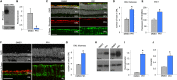
Results: In developing retina, PR1 causes a decrease in rod gene expression and an increase in S opsin+ cones. Photoregulin1 continues to inhibit rod gene expression in adult mice. When applied to two mouse models of RP, PR1 slows the degeneration of photoreceptors.
Conclusions: Chemical compounds identified as modulators of Nr2e3 activity may be useful for the treatment of RP through their effects on expression of disease-causing mutant genes.
Figures


Similar articles
-
Nuclear Receptor Subfamily 2 Group E Member 3 (NR2E3): Role in Retinal Development and Disease.Genes (Basel). 2023 Jun 23;14(7):1325. doi: 10.3390/genes14071325. Genes (Basel). 2023. PMID: 37510230 Free PMC article. Review.
-
Long-term preservation of cone photoreceptors and visual acuity in rd10 mutant mice exposed to continuous environmental enrichment.Mol Vis. 2014 Nov 5;20:1545-56. eCollection 2014. Mol Vis. 2014. PMID: 25489227 Free PMC article.
-
Large-scale phenotypic drug screen identifies neuroprotectants in zebrafish and mouse models of retinitis pigmentosa.Elife. 2021 Jun 29;10:e57245. doi: 10.7554/eLife.57245. Elife. 2021. PMID: 34184634 Free PMC article.
-
Different effects of valproic acid on photoreceptor loss in Rd1 and Rd10 retinal degeneration mice.Mol Vis. 2014 Nov 4;20:1527-44. eCollection 2014. Mol Vis. 2014. PMID: 25489226 Free PMC article.
-
NR2E3 mutations in enhanced S-cone sensitivity syndrome (ESCS), Goldmann-Favre syndrome (GFS), clumped pigmentary retinal degeneration (CPRD), and retinitis pigmentosa (RP).Hum Mutat. 2009 Nov;30(11):1475-85. doi: 10.1002/humu.21096. Hum Mutat. 2009. PMID: 19718767 Review.
Cited by
-
Interest rekindles in drug cocktails that reprogram cells.Nat Biotechnol. 2017 Jun 7;35(6):489-490. doi: 10.1038/nbt0617-489. Nat Biotechnol. 2017. PMID: 28591109 No abstract available.
-
Corneal cell therapy: with iPSCs, it is no more a far-sight.Stem Cell Res Ther. 2018 Oct 25;9(1):287. doi: 10.1186/s13287-018-1036-5. Stem Cell Res Ther. 2018. PMID: 30359313 Free PMC article. Review.
-
Nuclear Receptor Subfamily 2 Group E Member 3 (NR2E3): Role in Retinal Development and Disease.Genes (Basel). 2023 Jun 23;14(7):1325. doi: 10.3390/genes14071325. Genes (Basel). 2023. PMID: 37510230 Free PMC article. Review.
-
Nr2e3 is a genetic modifier that rescues retinal degeneration and promotes homeostasis in multiple models of retinitis pigmentosa.Gene Ther. 2021 May;28(5):223-241. doi: 10.1038/s41434-020-0134-z. Epub 2020 Mar 2. Gene Ther. 2021. PMID: 32123325 Free PMC article.
-
Cell Reprogramming for Regeneration and Repair of the Nervous System.Biomedicines. 2022 Oct 17;10(10):2598. doi: 10.3390/biomedicines10102598. Biomedicines. 2022. PMID: 36289861 Free PMC article. Review.
References
-
- Hartong DT,, Berson EL,, Dryja TP. Retinitis pigmentosa. Lancet. 2006; 368: 1795– 1809. - PubMed
-
- Nguyen AT,, Campbell M,, Kiang AS,, Humphries MM,, Humphries P. Current therapeutic strategies for P23H RHO-linked RP. Adv Exp Med Biol. 2014; 801: 471– 476. - PubMed
-
- Carter-Dawson LD,, LaVail MM. Rods and cones in the mouse retina. I. Structural analysis using light and electron microscopy. J Comp Neurol. 1979; 188 245– 262. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

