Sublethal exposure to alpha radiation (223Ra dichloride) enhances various carcinomas' sensitivity to lysis by antigen-specific cytotoxic T lymphocytes through calreticulin-mediated immunogenic modulation
- PMID: 27893426
- PMCID: PMC5341329
- DOI: 10.18632/oncotarget.13520
Sublethal exposure to alpha radiation (223Ra dichloride) enhances various carcinomas' sensitivity to lysis by antigen-specific cytotoxic T lymphocytes through calreticulin-mediated immunogenic modulation
Abstract
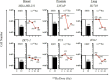
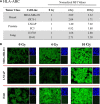
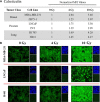
Radium-223 dichloride (Xofigo®; 223Ra) is an alpha-emitting radiopharmaceutical FDA-approved for the treatment of bone metastases in patients with advanced castration-resistant prostate cancer. It is also being examined clinically in patients with breast and lung carcinoma and patients with multiple myeloma. As with other forms of radiation, the aim of 223Ra is to reduce tumor burden by directly killing tumor cells. External beam (photon) and proton radiation have been shown to augment tumor sensitivity to antigen-specific CD8+ cytotoxic T lymphocytes (CTLs). However, little is known about whether treatment with 223Ra can also induce such immunogenic modulation in tumor cells that survive irradiation. We examined these effects in vitro by exposing human prostate, breast, and lung carcinoma cells to sublethal doses of 223Ra. 223Ra significantly enhanced T cell-mediated lysis of each tumor type by CD8+ CTLs specific for MUC-1, brachyury, and CEA tumor antigens. Immunofluorescence analysis revealed that the increase in CTL killing was accompanied by augmented protein expression of MHC-I and calreticulin in each tumor type, molecules that are essential for efficient antigen presentation. Enhanced tumor-cell lysis was facilitated by calreticulin surface translocation following 223Ra exposure. The phenotypic changes observed after treatment appear to be mediated by induction of the endoplasmic reticulum stress response pathway. By rendering tumor cells more susceptible to T cell-mediated lysis, 223Ra may potentially be effective in combination with various immunotherapies, particularly cancer vaccines that are designed to generate and expand patients' endogenous antigen-specific T-cell populations against specific tumor antigens.
Keywords: CTL-mediated lysis; alpha radiation; calreticulin; immunogenic modulation; radium-223.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

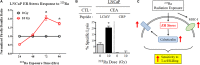
References
-
- Body JJ, Casimiro S, Costa L. Targeting bone metastases in prostate cancer: improving clinical outcome. Nature reviews Urology. 2015;12:340–356. - PubMed
-
- Ahmadzadehfar H, Eppard E, Kurpig S, Fimmers R, Yordanova A, Schlenkhoff CD, Gartner F, Rogenhofer S, Essler M. Therapeutic response and side effects of repeated radioligand therapy with 177Lu-PSMA-DKFZ-617 of castrate-resistant metastatic prostate cancer. Oncotarget. 2016;7:12477–12488. doi: 10.18632/oncotarget.7245. - DOI - PMC - PubMed
-
- Gartrell BA, Coleman R, Efstathiou E, Fizazi K, Logothetis CJ, Smith MR, Sonpavde G, Sartor O, Saad F. Metastatic Prostate Cancer and the Bone: Significance and Therapeutic Options. European urology. 2015;68:850–858. - PubMed
-
- Coleman R. Treatment of Metastatic Bone Disease and the Emerging Role of Radium-223. Seminars in nuclear medicine. 2016;46:99–104. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

