Cholesterol import and steroidogenesis are biosignatures for gastric cancer patient survival
- PMID: 27893427
- PMCID: PMC5352189
- DOI: 10.18632/oncotarget.13524
Cholesterol import and steroidogenesis are biosignatures for gastric cancer patient survival
Abstract
Androgens, estrogens, progesterone and related signals are reported to be involved in the pathology of gastric cancer. However, varied conclusions exist based on serum hormone levels, receptor expressions, and in vitro or in vivo studies. This report used a web-based gene survival analyzer to evaluate biochemical processes, including cholesterol importing via lipoprotein/receptors (L/R route), steroidogenic enzymes, and steroid receptors, in gastric cancer patients prognosis. The sex hormone receptors (androgen receptor, progesterone receptor, and estrogen receptor ESR1 or ESR2), L/R route (low/high-density lipoprotein receptors, LDLR/LRP6/SR-B1 and lipoprotein lipase, LPL) and steroidogenic enzymes (CYP11A1, HSD3B1, CYP17, HSD17B1, HSD3B1, CYP19A1 and SRD5A1) were associated with 5-year survival of gastric cancer patients. The AR, PR, ESR1 and ESR2 are progression promoters, as are the L/R route LDLR, LRP6, SR-B1 and LPL. It was found that CYP11A1, HSD3B1, CYP17, HSD17B1 and CYP19A1 promote progression, but dihydrotestosterone (DHT) converting enzyme SRD5A1 suppresses progression. Analyzing steroidogenic lipidome with a hazard ratio score algorithm found that CYP19A1 is the progression confounder in surgery, HER2 positive or negative patients. Finally, in the other patient cohort from TCGA, CYP19A1 was expressed higher in the tumor compared to that in normal counterparts, and also promoted progression. Lastly, exemestrane (type II aromatase inhibitor) dramatically suppress GCa cell growth in pharmacological tolerable doses in vitro. This work depicts a route-specific outside-in delivery of cholesterol to promote disease progression, implicating a host-to-tumor macroenvironmental regulation. The result indicating lipoprotein-mediated cholesterol entry and steroidogenesis are GCa progression biosignatures. And the exemestrane clinical trial in GCa patients of unmet medical needs is suggested.
Keywords: CYP19A1; cholesterol; gastric cancer; steroidogenesis.
Conflict of interest statement
The US and Taiwan patents regarding the use of exemestrane for gatric cancer therapy have been filed out, and WL Ma is the inventor. Other authors claim no interest conflict in this work.
Figures
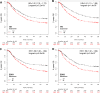
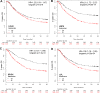




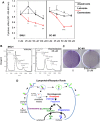
References
-
- Thrumurthy SG, Chaudry MA, Chau I, Allum W. Does surgery have a role in managing incurable gastric cancer? Nat Rev Clin Oncol. 2015;12:676–82. - PubMed
-
- Tan P, Yeoh KG. Genetics and Molecular Pathogenesis of Gastric Adenocarcinoma. Gastroenterology. 2015;149:1153–62. e3. - PubMed
-
- Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–97. - PubMed
-
- Allum WH, Blazeby JM, Griffin SM, Cunningham D, Jankowski JA, Wong R. Guidelines for the management of oesophageal and gastric cancer. Gut. 2011;60:1449–72. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

