Peripheral neuropathic pain: a mechanism-related organizing principle based on sensory profiles
- PMID: 27893485
- PMCID: PMC5266425
- DOI: 10.1097/j.pain.0000000000000753
Peripheral neuropathic pain: a mechanism-related organizing principle based on sensory profiles
Abstract
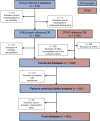
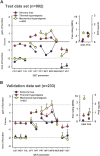
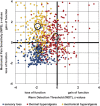
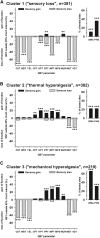
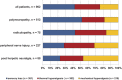
Patients with neuropathic pain are heterogeneous in etiology, pathophysiology, and clinical appearance. They exhibit a variety of pain-related sensory symptoms and signs (sensory profile). Different sensory profiles might indicate different classes of neurobiological mechanisms, and hence subgroups with different sensory profiles might respond differently to treatment. The aim of the investigation was to identify subgroups in a large sample of patients with neuropathic pain using hypothesis-free statistical methods on the database of 3 large multinational research networks (German Research Network on Neuropathic Pain (DFNS), IMI-Europain, and Neuropain). Standardized quantitative sensory testing was used in 902 (test cohort) and 233 (validation cohort) patients with peripheral neuropathic pain of different etiologies. For subgrouping, we performed a cluster analysis using 13 quantitative sensory testing parameters. Three distinct subgroups with characteristic sensory profiles were identified and replicated. Cluster 1 (sensory loss, 42%) showed a loss of small and large fiber function in combination with paradoxical heat sensations. Cluster 2 (thermal hyperalgesia, 33%) was characterized by preserved sensory functions in combination with heat and cold hyperalgesia and mild dynamic mechanical allodynia. Cluster 3 (mechanical hyperalgesia, 24%) was characterized by a loss of small fiber function in combination with pinprick hyperalgesia and dynamic mechanical allodynia. All clusters occurred across etiologies but frequencies differed. We present a new approach of subgrouping patients with peripheral neuropathic pain of different etiologies according to intrinsic sensory profiles. These 3 profiles may be related to pathophysiological mechanisms and may be useful in clinical trial design to enrich the study population for treatment responders.
Conflict of interest statement
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Figures
Comment in
-
Phenotypes and treatment response: it's difficult to make predictions, especially about the future.Pain. 2017 Feb;158(2):187-189. doi: 10.1097/j.pain.0000000000000771. Pain. 2017. PMID: 28092645 No abstract available.
References
-
- Attal N, de Andrade DC, Adam F, Ranoux D, Teixeira MJ, Galhardoni R, Raicher I, Uceyler N, Sommer C, Bouhassira D. Safety and efficacy of repeated injections of botulinum toxin A in peripheral neuropathic pain (BOTNEP): a randomised, double-blind, placebo-controlled trial. Lancet Neurol 2016;15:555–65. - PubMed
-
- Attal N, Fermanian C, Fermanian J, Lanteri-Minet M, Alchaar H, Bouhassira D. Neuropathic pain: are there distinct subtypes depending on the aetiology or anatomical lesion? PAIN 2008;138:343–53. - PubMed
-
- Attal N, Rouaud J, Brasseur L, Chauvin M, Bouhassira D. Systemic lidocaine in pain due to peripheral nerve injury and predictors of response. Neurology 2004;62:218–25. - PubMed
-
- Backonja MM, Attal N, Baron R, Bouhassira D, Drangholt M, Dyck PJ, Edwards RR, Freeman R, Gracely R, Haanpaa MH, Hansson P, Hatem SM, Krumova EK, Jensen TS, Maier C, Mick G, Rice AS, Rolke R, Treede RD, Serra J, Toelle T, Tugnoli V, Walk D, Walalce MS, Ware M, Yarnitsky D, Ziegler D. Value of quantitative sensory testing in neurological and pain disorders: NeuPSIG consensus. PAIN 2013;154:1807–19. - PubMed
-
- Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol 2010;9:807–19. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

