Outer membrane protein design
- PMID: 27894013
- PMCID: PMC5445000
- DOI: 10.1016/j.sbi.2016.11.003
Outer membrane protein design
Abstract
Membrane proteins are the gateway to the cell. These proteins are also a control center of the cell, as information from the outside is passed through membrane proteins as signals to the cellular machinery. The design of membrane proteins seeks to harness the power of these gateways and signal carriers. This review will focus on the design of the membrane proteins that are in the outer membrane, a membrane which only exists for gram negative bacteria, mitochondria, and chloroplasts. Unlike other membrane proteins, outer membrane proteins are uniquely shaped as β-barrels. Herein, I describe most known examples of membrane β-barrel design to date, focusing particularly on categorizing designs as: Firstly, structural deconstruction; secondly, structural changes; thirdly, chemical function design; and finally, the creation of new folds.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures
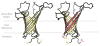
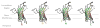

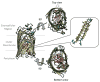
References
-
- Hagan CL, Silhavy TJ, Kahne D. β-barrel membrane protein assembly by the Bam complex. Annual Review of Biochemistry. 2011;80(1):189–210. - PubMed
-
- Osborne AR, Rapoport TA, Berg Bvd. Protein translocation by the Sec61/SecY channel. Annual Review of Cell and Developmental Biology. 2005;21(1):529–550. - PubMed
-
- Senes A. Computational design of membrane proteins. Current Opinion in Structural Biology. 2011;21(4):460–466. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

