A novel fully human anti-NCL immunoRNase for triple-negative breast cancer therapy
- PMID: 27894092
- PMCID: PMC5349967
- DOI: 10.18632/oncotarget.13522
A novel fully human anti-NCL immunoRNase for triple-negative breast cancer therapy
Abstract
Breast cancer is the most common cancer in women worldwide. A new promising anti-cancer therapy involves the use of monoclonal antibodies specific for target tumor-associated antigens (TAAs). A TAA of interest for immunotherapy of Triple Negative Breast Cancer (TNBC) is nucleolin (NCL), a multifunctional protein, selectively expressed on the surface of cancer cells, which regulates the biogenesis of specific microRNAs (miRNAs) involved in tumor development and drug-resistance. We previously isolated a novel human anti-NCL scFv, called 4LB5, that is endowed with selective anti-tumor effects. Here we report the construction and characterization of a novel immunoRNase constituted by 4LB5 and a human pancreatic RNase (HP-RNase) called "4LB5-HP-RNase". This immunoRNase retains both the enzymatic activity of human pancreatic RNase and the specific binding of the parental scFv to a panel of surface NCL-positive breast cancer cells. Notably, 4LB5-HP-RNase dramatically and selectively reduced the viability and proliferation of NCL-positive tumor cells in vitro and in vivo. Specifically, it induced apoptosis and reduced the levels of the tumorigenic miRNAs miR-21, -221 and -222. Thus, this novel immunoagent could be a valuable tool for the treatment of TNBC patients ineligible for currently available targeted treatments.
Keywords: cancer immunotherapy; human RNase; microRNA; nucleolin; triple negative breast cancer.
Conflict of interest statement
The authors declare as a potential conflict of interest that a patent application including previous work on 4LB5 has been filed.
Figures
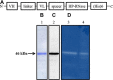

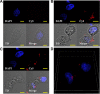
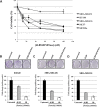

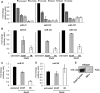
References
-
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. - PubMed
-
- Stebbing J, Copson E, O’Reilly S. Herceptin (trastuzumab) in advanced breast cancer. Cancer Treat Rev. 2000;26:287–90. - PubMed
-
- Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, Swain SM, Pisansky TM, Fehrenbacher L, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–84. - PubMed
-
- Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–12. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

