Gene cassette knock-in in mammalian cells and zygotes by enhanced MMEJ
- PMID: 27894274
- PMCID: PMC5126809
- DOI: 10.1186/s12864-016-3331-9
Gene cassette knock-in in mammalian cells and zygotes by enhanced MMEJ
Abstract
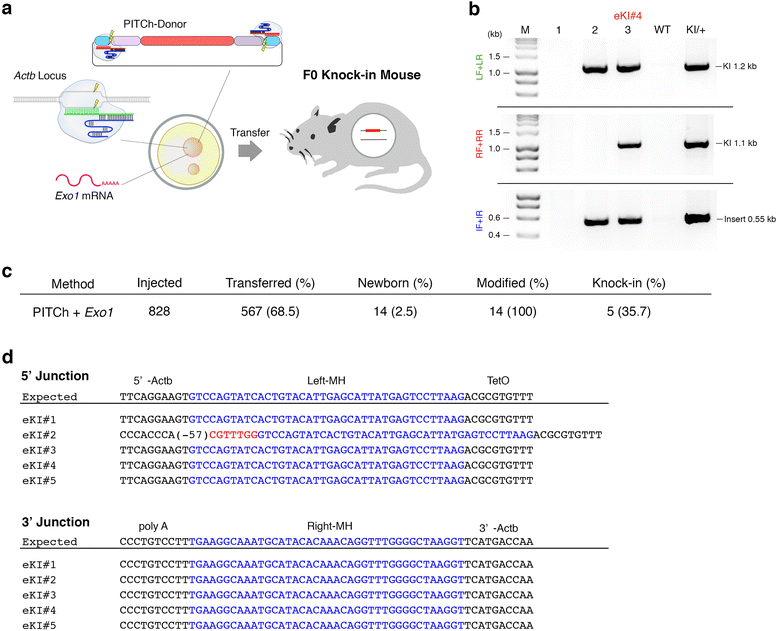
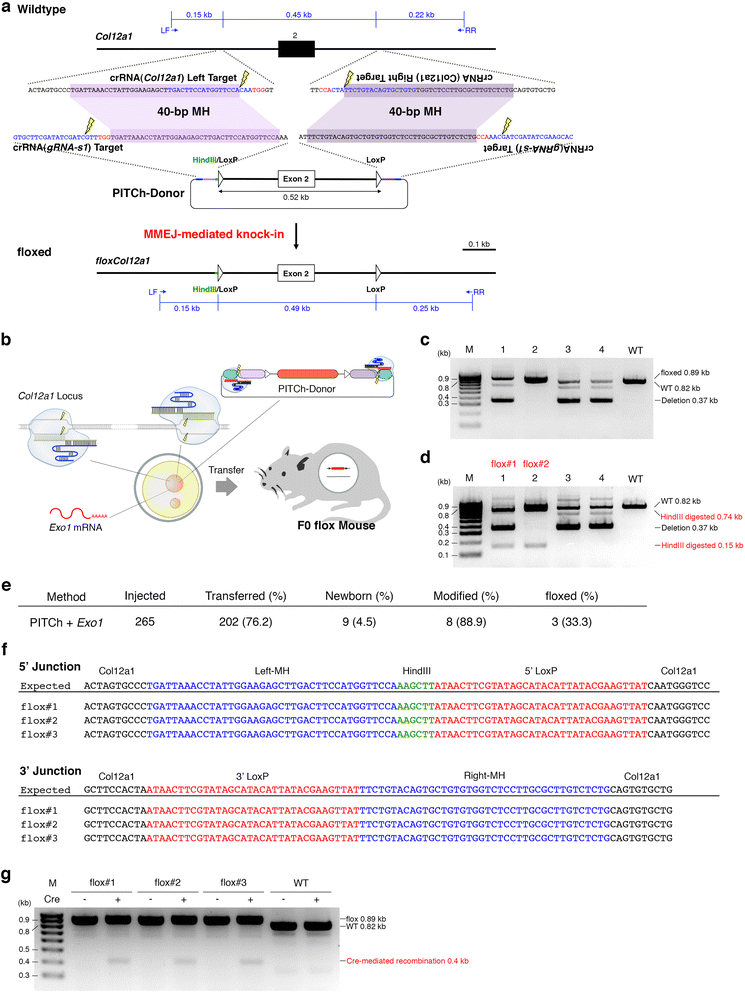
Background: Although CRISPR/Cas enables one-step gene cassette knock-in, assembling targeting vectors containing long homology arms is a laborious process for high-throughput knock-in. We recently developed the CRISPR/Cas-based precise integration into the target chromosome (PITCh) system for a gene cassette knock-in without long homology arms mediated by microhomology-mediated end-joining.
Results: Here, we identified exonuclease 1 (Exo1) as an enhancer for PITCh in human cells. By combining the Exo1 and PITCh-directed donor vectors, we achieved convenient one-step knock-in of gene cassettes and floxed allele both in human cells and mouse zygotes.
Conclusions: Our results provide a technical platform for high-throughput knock-in.
Keywords: CRISPR/Cas; Cloning-free; Exo1; Flox; Gene cassette; High throughput; Knock-in; MMEJ; Mouse; Reporter.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

