Intestinal dysbiosis is common in systemic sclerosis and associated with gastrointestinal and extraintestinal features of disease
- PMID: 27894337
- PMCID: PMC5126986
- DOI: 10.1186/s13075-016-1182-z
Intestinal dysbiosis is common in systemic sclerosis and associated with gastrointestinal and extraintestinal features of disease
Abstract
Background: Recent evidence suggests a link between autoimmunity and the intestinal microbial composition in several rheumatic diseases including systemic sclerosis (SSc). The objective of this study was to investigate the prevalence of intestinal dysbiosis in SSc and to characterise patients suffering from this potentially immunomodulatory deviation.
Methods: This study consisted of 98 consecutive patients subject to in-hospital care. Stool samples were analysed for intestinal microbiota composition using a validated genome-based microbiota test (GA-map™ Dysbiosis Test, Genetic Analysis, Oslo, Norway). Gut microbiota dysbiosis was found present as per this standardised test. Patients were examined regarding gastrointestinal and extraintestinal manifestations of SSc by clinical, laboratory, and radiological measures including esophageal cineradiography, the Malnutrition Universal Screening Tool (MUST), levels of plasma transthyretin (a marker of malnutrition) and faecal (F-) calprotectin (a marker of intestinal inflammation).
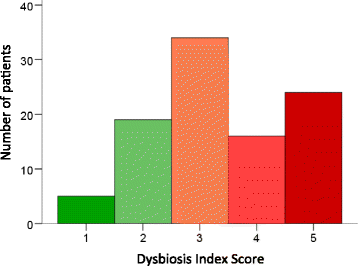
Results: A majority (75.5%) of the patients exhibited dysbiosis. Dysbiosis was more severe (rs = 0.31, p = 0.001) and more common (p = 0.013) in patients with esophageal dysmotility. Dysbiosis was also more pronounced in patients with abnormal plasma levels of transthyretin (p = 0.045) or micronutrient deficiency (p = 0.009). In 19 patients at risk for malnutrition according to the MUST, 18 exhibited dysbiosis. Conversely, of the 24 patients with a negative dysbiosis test, only one was at risk for malnutrition. The mean ± SEM levels of F-calprotectin were 112 ± 14 and 45 ± 8 μg/g in patients with a positive and negative dysbiosis test, respectively. Dysbiosis was more severe in patients with skin telangiectasias (p = 0.020), pitting scars (p = 0.023), pulmonary fibrosis (p = 0.009), and elevated serum markers of inflammation (p < 0.001). However, dysbiosis did not correlate with age, disease duration, disease subtype, or extent of skin fibrosis.
Conclusions: In this cross-sectional study, intestinal dysbiosis was common in patients with SSc and was associated with gastrointestinal dysfunction, malnutrition and with some inflammatory, fibrotic and vascular extraintestinal features of SSc. Further studies are needed to elucidate the potential causal relationship of intestinal microbe-host interaction in this autoimmune disease.
Keywords: Dysbiosis; Gastrointestinal; Microbiome; Systemic sclerosis.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

