Vancomycin and the Risk of AKI: A Systematic Review and Meta-Analysis
- PMID: 27895134
- PMCID: PMC5142072
- DOI: 10.2215/CJN.05920616
Vancomycin and the Risk of AKI: A Systematic Review and Meta-Analysis
Abstract
Background and objectives: Vancomycin has been in use for more than half a century, but whether it is truly nephrotoxic and to what extent are still highly controversial. The objective of this study was to determine the risk of AKI attributable to intravenous vancomycin.
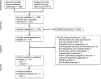
Design, setting, participants, & measurements: We conducted a systematic review of randomized, controlled trials and cohort studies that compared patients treated with intravenous vancomycin with a control group of patients given a comparator nonglycopeptide antibiotic and in which kidney function or kidney injury outcomes were reported. PubMed and Cochrane Library were searched from 1990 to September of 2015. Two reviewers extracted data and assessed study risk of bias, and one reviewer adjudicated the assessments. A meta-analysis was conducted on seven randomized, controlled trials (total of 4033 patients).
Results: Moderate quality evidence suggested that vancomycin treatment is associated with a higher risk of AKI, with a relative risk of 2.45 (95% confidence interval, 1.69 to 3.55). The risk of kidney injury was similar in patients treated for skin and soft tissue infections compared with those treated for nosocomial pneumonia and other complicated infections. There was an uncertain risk of reporting bias, because kidney function was not a prespecified outcome in any of the trials. The preponderance of evidence was judged to be indirect, because the majority of studies compared vancomycin specifically with linezolid.
Conclusions: Our findings suggest that there is a measurable risk of AKI associated with vancomycin, but the strength of the evidence is moderate. A randomized, controlled trial designed to study kidney function as an outcome would be needed to draw unequivocal conclusions.
Keywords: Anti-Bacterial Agents; Cohort Studies; Control Groups; Cross Infection; Glycopeptides; Humans; Libraries; Linezolid; Pneumonia; PubMed; Randomized Controlled Trials as Topic; Risk Assessment; Soft Tissue Infections; Vancomycin; acute kidney injury; chronic renal insufficiency; meta-analysis.
Copyright © 2016 by the American Society of Nephrology.
Figures


Comment in
-
Vancomycin and the Risk of AKI: Now Clearer than Mississippi Mud.Clin J Am Soc Nephrol. 2016 Dec 7;11(12):2101-2103. doi: 10.2215/CJN.11011016. Epub 2016 Nov 28. Clin J Am Soc Nephrol. 2016. PMID: 27895133 Free PMC article. No abstract available.
References
-
- Bailie GR, Neal D: Vancomycin ototoxicity and nephrotoxicity. A review. Med Toxicol 3: 376–386, 1988 - PubMed
-
- Cheung RP, DiPiro JT: Vancomycin: An update. Pharmacotherapy 6: 153–169, 1986 - PubMed
-
- Nishino Y, Takemura S, Minamiyama Y, Hirohashi K, Ogino T, Inoue M, Okada S, Kinoshita H: Targeting superoxide dismutase to renal proximal tubule cells attenuates vancomycin-induced nephrotoxicity in rats. Free Radic Res 37: 373–379, 2003 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

