The E Loop of the Transmitter Binding Site Is a Key Determinant of the Modulatory Effects of Physostigmine on Neuronal Nicotinic α4β2 Receptors
- PMID: 27895161
- PMCID: PMC5267520
- DOI: 10.1124/mol.116.106484
The E Loop of the Transmitter Binding Site Is a Key Determinant of the Modulatory Effects of Physostigmine on Neuronal Nicotinic α4β2 Receptors
Abstract
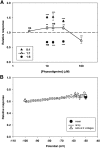
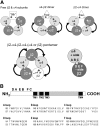
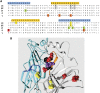
Physostigmine is a well known inhibitor of acetylcholinesterase, which can also activate, potentiate, and inhibit acetylcholine receptors, including neuronal nicotinic receptors comprising α4 and β2 subunits. We have found that the two stoichiometric forms of this receptor differ in the effects of physostigmine. The form containing three copies of α4 and two of β2 was potentiated at low concentrations of acetylcholine chloride (ACh) and physostigmine, whereas the form containing two copies of α4 and three of β2 was inhibited. Chimeric constructs of subunits indicated that the presence of inhibition or potentiation depended on the source of the extracellular ligand binding domain of the subunit. Further sets of chimeric constructs demonstrated that a portion of the ACh binding domain, the E loop, is a key determinant. Transferring the E loop from the β2 subunit to the α4 subunit resulted in strong inhibition, whereas the reciprocal transfer reduced inhibition. To control the number and position of the incorporated chimeric subunits, we expressed chimeric constructs with subunit dimers. Surprisingly, incorporation of a subunit with an altered E loop had similar effects whether it contributed either to an intersubunit interface containing a canonical ACh binding site or to an alternative interface. The observation that the α4 E loop is involved suggests that physostigmine interacts with regions of subunits that contribute to the ACh binding site, whereas the lack of interface specificity indicates that interaction with a particular ACh binding site is not the critical factor.
Copyright © 2016 by The American Society for Pharmacology and Experimental Therapeutics.
Figures

References
-
- Cooper JC, Gutbrod O, Witzemann V, Methfessel C. (1996) Pharmacology of the nicotinic acetylcholine receptor from fetal rat muscle expressed in Xenopus oocytes. Eur J Pharmacol 309:287–298. - PubMed
-
- Dani JA, Bertrand D. (2007) Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol 47:699–729. - PubMed
-
- Gotti C, Moretti M, Gaimarri A, Zanardi A, Clementi F, Zoli M. (2007) Heterogeneity and complexity of native brain nicotinic receptors. Biochem Pharmacol 74:1102–1111. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

