Introduction of an N-Glycosylation Site into UDP-Glucuronosyltransferase 2B3 Alters Its Sensitivity to Cytochrome P450 3A1-Dependent Modulation
- PMID: 27895582
- PMCID: PMC5107996
- DOI: 10.3389/fphar.2016.00427
Introduction of an N-Glycosylation Site into UDP-Glucuronosyltransferase 2B3 Alters Its Sensitivity to Cytochrome P450 3A1-Dependent Modulation
Abstract
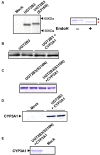
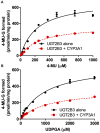
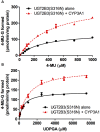
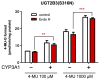
Our previous studies have demonstrated functional protein-protein interactions between cytochrome P450 (CYP) 3A and UDP-glucuronosyltransferase (UGT). However, the role of carbohydrate chains of UGTs in the interaction with CYP is not well understood. To address this issue, we examined whether CYP3A1 modulates the function of UGT2B3 which lacks potential glycosylation sites. We also examined whether the introduction of N-glycosylation to UGT2B3 affects CYP3A-dependent modulation of UGT function. To introduce a potential glycosylation site into UGT2B3, Ser 316 of UGT2B3 was substituted with Asn by site-directed mutagenesis. A baculovirus-Sf-9 cell system for expressing CYP3A1 and UGT2B3/UGT2B3(S316N) was established using a Bac-to-Bac system. Glycosylation of UGT2B3(S316N) was demonstrated in this expression system. The microsomal activity of recombinant UGT was determined using 4-methylumbelliferone as a substrate. The effect of CYP3A1 co-expression on UGT function was examined by comparing the kinetic profiles between single (UGT alone) and double expression (UGT plus CYP) systems. The kinetics of the two expression systems fitted a Michaelis-Menten equation. When the 4-MU concentration was varied, co-expression of CYP3A1 lowered the Vmax of UGT2B3-mediated conjugation. Conversely, for UGT2B3(S316N), the Vmax in the dual expression system was higher than that in the single expression system. The data obtained demonstrate that the introduction of N-glycosylation to UGT2B3 alters its sensitivity to CYP3A1-dependent modulation while CYP3A1 enhanced UGT2B3(S316N) activity, and wild-type UGT2B3 was suppressed by CYP3A1. These data suggest that N-glycosylation of UGT is one of the determinants regulating the interaction between CYP3A and UGT.
Keywords: CYP; P450; UDP-glucuronosyltransferase; UGT; cytochrome P450; protein–protein interaction.
Figures


References
-
- Gonzalez F. J., Nebert D. W., Hardwick J. P., Kasper C. B. (1985). Complete cDNA and protein sequence of a pregnenolone 16 alpha-carbonitrile-induced cytochrome P-450. A representative of a new gene family. J. Biol. Chem. 260 7435–7441. - PubMed
-
- Hanioka N., Jinno H., Nishimura T., Ando M., Ozawa S., Sawada J. (2001). High-performance liquid chromatographic assay for glucuronidation activity of 7-ethyl-10-hydroxycamptothecin (SN-38), the active metabolite of irinotecan (CPT-11), in human liver microsomes. Biomed. Chromatogr. 15 328–333. 10.1002/bmc.76 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

