Rapid Evolution of Manifold CRISPR Systems for Plant Genome Editing
- PMID: 27895652
- PMCID: PMC5107562
- DOI: 10.3389/fpls.2016.01683
Rapid Evolution of Manifold CRISPR Systems for Plant Genome Editing
Abstract
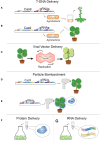
Advanced CRISPR-Cas9 based technologies first validated in mammalian cell systems are quickly being adapted for use in plants. These new technologies increase CRISPR-Cas9's utility and effectiveness by diversifying cellular capabilities through expression construct system evolution and enzyme orthogonality, as well as enhanced efficiency through delivery and expression mechanisms. Here, we review the current state of advanced CRISPR-Cas9 and Cpf1 capabilities in plants and cover the rapid evolution of these tools from first generation inducers of double strand breaks for basic genetic manipulations to second and third generation multiplexed systems with myriad functionalities, capabilities, and specialized applications. We offer perspective on how to utilize these tools for currently untested research endeavors and analyze strengths and weaknesses of novel CRISPR systems in plants. Advanced CRISPR functionalities and delivery options demonstrated in plants are primarily reviewed but new technologies just coming to the forefront of CRISPR development, or those on the horizon, are briefly discussed. Topics covered are focused on the expansion of expression and delivery capabilities for CRISPR-Cas9 components and broadening targeting range through orthogonal Cas9 and Cpf1 proteins.
Keywords: CRISPR; Cas9; Cpf1; RNA-guided endonucleases; plant genome editing; sequence specific nucleases.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

