Deciphering fact from artifact when using reporter assays to investigate the roles of host factors on L1 retrotransposition
- PMID: 27895722
- PMCID: PMC5120415
- DOI: 10.1186/s13100-016-0079-3
Deciphering fact from artifact when using reporter assays to investigate the roles of host factors on L1 retrotransposition
Abstract
Background: The Long INterspersed Element-1 (L1, LINE-1) is the only autonomous mobile DNA element in humans and has generated as much as half of the genome. Due to increasing clinical interest in the roles of L1 in cancer, embryogenesis and neuronal development, it has become a priority to understand L1-host interactions and identify host factors required for its activity. Apropos to this, we recently reported that L1 retrotransposition in HeLa cells requires phosphorylation of the L1 protein ORF1p at motifs targeted by host cell proline-directed protein kinases (PDPKs), which include the family of mitogen-activated protein kinases (MAPKs). Using two engineered L1 reporter assays, we continued our investigation into the roles of MAPKs in L1 activity.
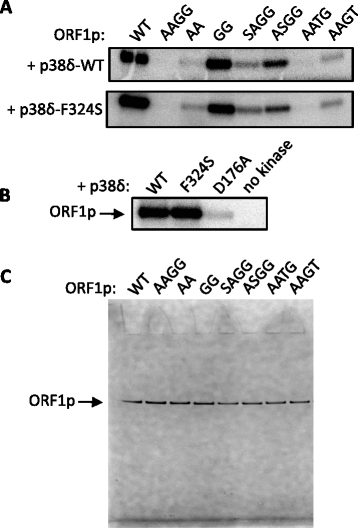
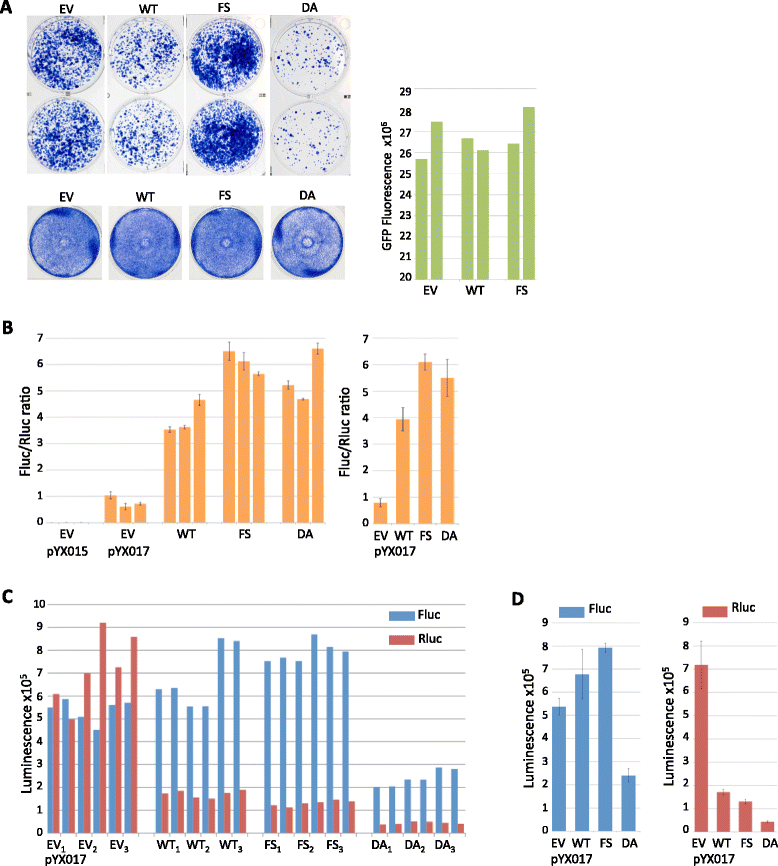
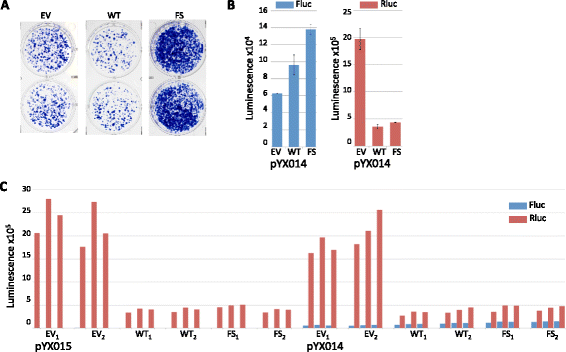
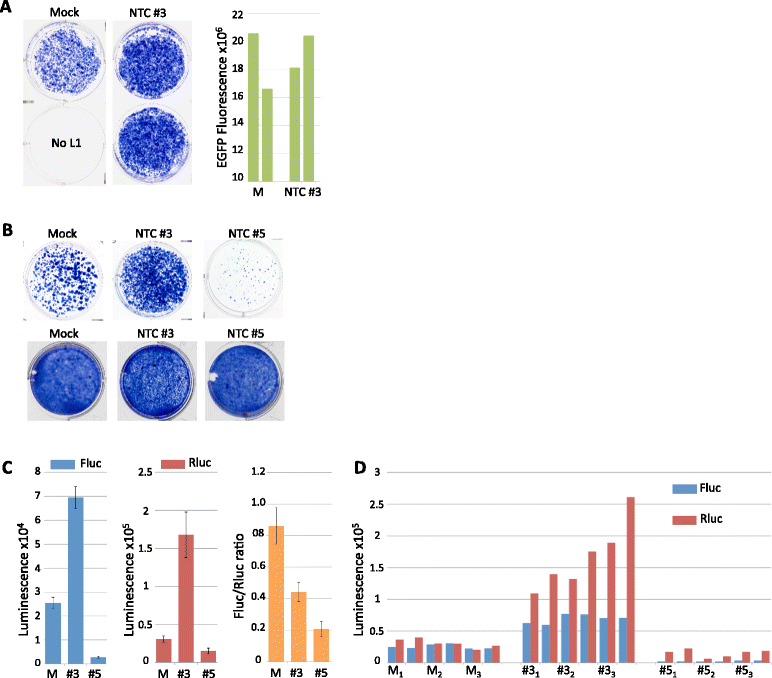
Results: We found that the MAPK p38δ phosphorylated ORF1p on three of its four PDPK motifs required for L1 activity. In addition, we found that a constitutively active p38δ mutant appeared to promote L1 retrotransposition in HeLa cells. However, despite the consistency of these findings with our earlier work, we identified some technical concerns regarding the experimental methodology. Specifically, we found that exogenous expression of p38δ appeared to affect at least one heterologous promoter in an engineered L1 reporter, as well as generate opposing effects on two different reporters. We also show that two commercially available non-targeting control (NTC) siRNAs elicit drastically different effects on the apparent retrotransposition reported by both L1 assays, which raises concerns about the use of NTCs as normalizing controls.
Conclusions: Engineered L1 reporter assays have been invaluable for determining the functions and critical residues of L1 open reading frames, as well as elucidating many aspects of L1 replication. However, our results suggest that caution is required when interpreting data obtained from L1 reporters used in conjunction with exogenous gene expression or siRNA.
Keywords: HSV-TK; Host factor; L1; LINE-1; Promoter; Renilla; Reporter; SV40; p38.
Figures


Similar articles
-
Phosphorylation of ORF1p is required for L1 retrotransposition.Proc Natl Acad Sci U S A. 2015 Apr 7;112(14):4298-303. doi: 10.1073/pnas.1416869112. Epub 2015 Mar 23. Proc Natl Acad Sci U S A. 2015. PMID: 25831499 Free PMC article.
-
The Zinc-Finger Antiviral Protein ZAP Inhibits LINE and Alu Retrotransposition.PLoS Genet. 2015 May 7;11(5):e1005121. doi: 10.1371/journal.pgen.1005121. eCollection 2015 May. PLoS Genet. 2015. PMID: 25951186 Free PMC article.
-
The RNA polymerase dictates ORF1 requirement and timing of LINE and SINE retrotransposition.PLoS Genet. 2009 Apr;5(4):e1000458. doi: 10.1371/journal.pgen.1000458. Epub 2009 Apr 24. PLoS Genet. 2009. PMID: 19390602 Free PMC article.
-
Guardian of the Human Genome: Host Defense Mechanisms against LINE-1 Retrotransposition.Front Chem. 2016 Jun 28;4:28. doi: 10.3389/fchem.2016.00028. eCollection 2016. Front Chem. 2016. PMID: 27446907 Free PMC article. Review.
-
Post-Transcriptional Control of LINE-1 Retrotransposition by Cellular Host Factors in Somatic Cells.Front Cell Dev Biol. 2016 Mar 7;4:14. doi: 10.3389/fcell.2016.00014. eCollection 2016. Front Cell Dev Biol. 2016. PMID: 27014690 Free PMC article. Review.
Cited by
-
sRNA/L1 retrotransposition: using siRNAs and miRNAs to expand the applications of the cell culture-based LINE-1 retrotransposition assay.Philos Trans R Soc Lond B Biol Sci. 2020 Mar 30;375(1795):20190346. doi: 10.1098/rstb.2019.0346. Epub 2020 Feb 10. Philos Trans R Soc Lond B Biol Sci. 2020. PMID: 32075559 Free PMC article.
-
Dynamic silencing of somatic L1 retrotransposon insertions reflects the developmental and cellular contexts of their genomic integration.Mob DNA. 2017 May 10;8:8. doi: 10.1186/s13100-017-0091-2. eCollection 2017. Mob DNA. 2017. PMID: 28491150 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

