Validation of biomarkers to predict response to immunotherapy in cancer: Volume I - pre-analytical and analytical validation
- PMID: 27895917
- PMCID: PMC5109744
- DOI: 10.1186/s40425-016-0178-1
Validation of biomarkers to predict response to immunotherapy in cancer: Volume I - pre-analytical and analytical validation
Abstract
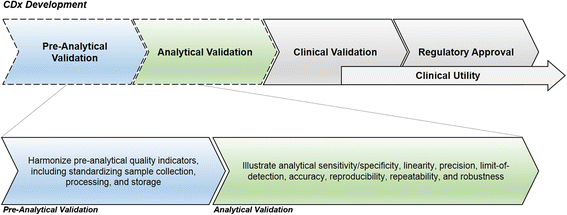
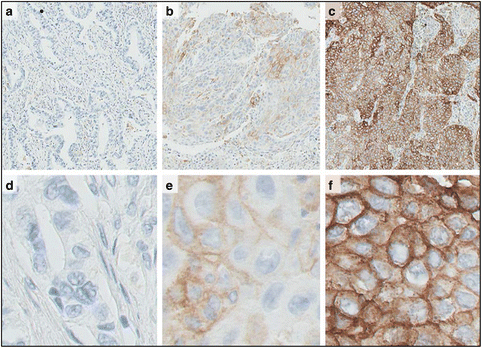
Immunotherapies have emerged as one of the most promising approaches to treat patients with cancer. Recently, there have been many clinical successes using checkpoint receptor blockade, including T cell inhibitory receptors such as cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and programmed cell death-1 (PD-1). Despite demonstrated successes in a variety of malignancies, responses only typically occur in a minority of patients in any given histology. Additionally, treatment is associated with inflammatory toxicity and high cost. Therefore, determining which patients would derive clinical benefit from immunotherapy is a compelling clinical question. Although numerous candidate biomarkers have been described, there are currently three FDA-approved assays based on PD-1 ligand expression (PD-L1) that have been clinically validated to identify patients who are more likely to benefit from a single-agent anti-PD-1/PD-L1 therapy. Because of the complexity of the immune response and tumor biology, it is unlikely that a single biomarker will be sufficient to predict clinical outcomes in response to immune-targeted therapy. Rather, the integration of multiple tumor and immune response parameters, such as protein expression, genomics, and transcriptomics, may be necessary for accurate prediction of clinical benefit. Before a candidate biomarker and/or new technology can be used in a clinical setting, several steps are necessary to demonstrate its clinical validity. Although regulatory guidelines provide general roadmaps for the validation process, their applicability to biomarkers in the cancer immunotherapy field is somewhat limited. Thus, Working Group 1 (WG1) of the Society for Immunotherapy of Cancer (SITC) Immune Biomarkers Task Force convened to address this need. In this two volume series, we discuss pre-analytical and analytical (Volume I) as well as clinical and regulatory (Volume II) aspects of the validation process as applied to predictive biomarkers for cancer immunotherapy. To illustrate the requirements for validation, we discuss examples of biomarker assays that have shown preliminary evidence of an association with clinical benefit from immunotherapeutic interventions. The scope includes only those assays and technologies that have established a certain level of validation for clinical use (fit-for-purpose). Recommendations to meet challenges and strategies to guide the choice of analytical and clinical validation design for specific assays are also provided.
Keywords: Assay; Biomarker; Cancer; Immunotherapy; Standardization; Validation.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
