Optical redox ratio identifies metastatic potential-dependent changes in breast cancer cell metabolism
- PMID: 27895979
- PMCID: PMC5119579
- DOI: 10.1364/BOE.7.004364
Optical redox ratio identifies metastatic potential-dependent changes in breast cancer cell metabolism
Abstract
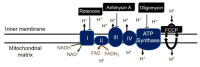
The development of prognostic indicators of breast cancer metastatic risk could reduce the number of patients receiving chemotherapy for tumors with low metastatic potential. Recent evidence points to a critical role for cell metabolism in driving breast cancer metastasis. Endogenous fluorescence intensity of nicotinamide adenine dinucleotide (NADH) and flavin adenine dinucleotide (FAD) can provide a label-free method for assessing cell metabolism. We report the optical redox ratio of FAD/(FAD + NADH) of four isogenic triple-negative breast cancer cell lines with varying metastatic potential. Under normoxic conditions, the redox ratio increases with increasing metastatic potential (168FARN>4T07>4T1), indicating a shift to more oxidative metabolism in cells capable of metastasis. Reoxygenation following acute hypoxia increased the redox ratio by 43 ± 9% and 33 ± 4% in the 4T1 and 4T07 cells, respectively; in contrast, the redox ratio decreased 14 ± 7% in the non-metastatic 67NR cell line. These results demonstrate that the optical redox ratio is sensitive to the metabolic adaptability of breast cancer cells with high metastatic potential and could potentially be used to measure dynamic functional changes that are indicative of invasive or metastatic potential.
Keywords: (170.1530) Cell analysis; (170.2520) Fluorescence microscopy; (170.2655) Functional monitoring and imaging.
Figures
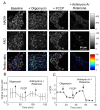
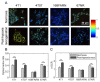
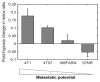
References
-
- Henry N. L., Somerfield M. R., Abramson V. G., Allison K. H., Anders C. K., Chingos D. T., Hurria A., Openshaw T. H., Krop I. E., “Role of Patient and Disease Factors in Adjuvant Systemic Therapy Decision Making for Early-Stage, Operable Breast Cancer: American Society of Clinical Oncology Endorsement of Cancer Care Ontario Guideline Recommendations,” J. Clin. Oncol. 34(19), 2303–2311 (2016). 10.1200/JCO.2015.65.8609 - DOI - PubMed
-
- Peto R., Davies C., Godwin J., Gray R., Pan H. C., Clarke M., Cutter D., Darby S., McGale P., Taylor C., Wang Y. C., Bergh J., Di Leo A., Albain K., Swain S., Piccart M., Pritchard K., Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) , “Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials,” Lancet 379(9814), 432–444 (2012). 10.1016/S0140-6736(11)61625-5 - DOI - PMC - PubMed
-
- Cronin M., Sangli C., Liu M.-L., Pho M., Dutta D., Nguyen A., Jeong J., Wu J., Langone K. C., Watson D., “Analytical Validation of the Oncotype DX Genomic Diagnostic Test for Recurrence Prognosis and Therapeutic Response Prediction in Node-Negative, Estrogen Receptor-Positive Breast Cancer,” Clin. Chem. 53(6), 1084–1091 (2007). 10.1373/clinchem.2006.076497 - DOI - PubMed
-
- Warburg O., Posener K., Negelein E., “The metabolism of the carcinoma cell,” The Metabolism of Tumors. New York, Richard R. Smith, Inc, 29169 (1931).
-
- Lin X., Zhang F., Bradbury C. M., Kaushal A., Li L., Spitz D. R., Aft R. L., Gius D., “2-Deoxy-D-glucose-induced cytotoxicity and radiosensitization in tumor cells is mediated via disruptions in thiol metabolism,” Cancer Res. 63(12), 3413–3417 (2003). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
