Rapid mesoscale multiphoton microscopy of human skin
- PMID: 27895980
- PMCID: PMC5119580
- DOI: 10.1364/BOE.7.004375
Rapid mesoscale multiphoton microscopy of human skin
Abstract
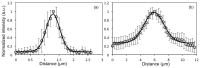
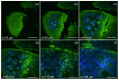
We present a multiphoton microscope designed for mesoscale imaging of human skin. The system is based on two-photon excited fluorescence and second-harmonic generation, and images areas of ~0.8x0.8 mm2 at speeds of 0.8 fps (800x800 pixels; 12 frame averages) for high signal-to-noise ratio, with lateral and axial resolutions of 0.5µm and 3.3µm, respectively. The main novelty of this instrument is the design of the scan head, which includes a fast galvanometric scanner, optimized relay optics, a beam expander and high NA objective lens. Computed aberrations in focus are below the Marechal criterion of 0.07λ rms for diffraction-limited performance. We demonstrate the practical utility of this microscope by ex-vivo imaging of wide areas in normal human skin.
Keywords: (170.2520) Fluorescence microscopy; (180.0180) Microscopy; (180.4315) Nonlinear microscopy.
Figures






References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
