Residual OCTN2 transporter activity, carnitine levels and symptoms correlate in patients with primary carnitine deficiency
- PMID: 27896095
- PMCID: PMC5121291
- DOI: 10.1016/j.ymgmr.2014.04.008
Residual OCTN2 transporter activity, carnitine levels and symptoms correlate in patients with primary carnitine deficiency
Abstract
Background: The prevalence of primary carnitine deficiency (PCD) in the Faroe Islands is the highest reported in the world (1:300). Serious symptoms related to PCD, e.g. sudden death, have previously only been associated to the c.95A > G/c.95A > G genotype in the Faroe Islands. We report and characterize novel mutations associated with PCD in the Faroese population and report and compare free carnitine levels and OCTN2 transport activities measured in fibroblasts from PCD patients with different genotypes.
Methods: Genetic analyses were used to identify novel mutations, and carnitine uptake analyses in cultured skin fibroblasts from selected patients were used to examine residual OCTN2 transporter activities of the various genotypes.
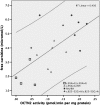
Results: Four different mutations, including the unpublished c.131C > T (p.A44V), the novel splice mutation c.825-52G > A and a novel risk-haplotype (RH) were identified in the Faroese population. The two most prevalent genotypes were c.95A > G/RH (1:600) and c.95A > G/c.95A > G (1:1300). Patients homozygous for the c.95A > G mutation had both the significantly (p < 0.01) lowest mean free carnitine level at 2.03 (SD 0.66) μmol/L and lowest residual OCTN2 transporter activity (4% of normal). There was a significant positive correlation between free carnitine levels and residual OCTN2 transporter activities in PCD patients (R2 = 0.430, p < 0.01).
Conclusion: There was a significant positive correlation between carnitine levels and OCTN2 transporter activities. The c.95A > G/c.95A > G genotype had the significantly lowest mean free carnitine level and residual OCTN2 transporter activity.
Keywords: OCTN2; Primary carnitine deficiency; SLC22A5; The Faroe Islands.
Figures
Similar articles
-
Carnitine transport and fatty acid oxidation.Biochim Biophys Acta. 2016 Oct;1863(10):2422-35. doi: 10.1016/j.bbamcr.2016.01.023. Epub 2016 Jan 29. Biochim Biophys Acta. 2016. PMID: 26828774 Free PMC article. Review.
-
Carnitine levels in skeletal muscle, blood, and urine in patients with primary carnitine deficiency during intermission of L-carnitine supplementation.JIMD Rep. 2015;20:103-11. doi: 10.1007/8904_2014_398. Epub 2015 Feb 10. JIMD Rep. 2015. PMID: 25665836 Free PMC article.
-
Carnitine levels in 26,462 individuals from the nationwide screening program for primary carnitine deficiency in the Faroe Islands.J Inherit Metab Dis. 2014 Mar;37(2):215-22. doi: 10.1007/s10545-013-9606-2. Epub 2013 May 8. J Inherit Metab Dis. 2014. PMID: 23653224
-
Carnitine levels and mutations in the SLC22A5 gene in Faroes patients with Parkinson's disease.Neurosci Lett. 2018 May 14;675:116-119. doi: 10.1016/j.neulet.2018.03.064. Epub 2018 Mar 31. Neurosci Lett. 2018. PMID: 29614331
-
Pharmacological and pathophysiological roles of carnitine/organic cation transporters (OCTNs: SLC22A4, SLC22A5 and Slc22a21).Biopharm Drug Dispos. 2013 Jan;34(1):29-44. doi: 10.1002/bdd.1816. Epub 2012 Oct 14. Biopharm Drug Dispos. 2013. PMID: 22952014 Review.
Cited by
-
Carnitine transport and fatty acid oxidation.Biochim Biophys Acta. 2016 Oct;1863(10):2422-35. doi: 10.1016/j.bbamcr.2016.01.023. Epub 2016 Jan 29. Biochim Biophys Acta. 2016. PMID: 26828774 Free PMC article. Review.
-
Carnitine levels in skeletal muscle, blood, and urine in patients with primary carnitine deficiency during intermission of L-carnitine supplementation.JIMD Rep. 2015;20:103-11. doi: 10.1007/8904_2014_398. Epub 2015 Feb 10. JIMD Rep. 2015. PMID: 25665836 Free PMC article.
-
Functional genomics of OCTN2 variants informs protein-specific variant effect predictor for Carnitine Transporter Deficiency.Proc Natl Acad Sci U S A. 2022 Nov 16;119(46):e2210247119. doi: 10.1073/pnas.2210247119. Epub 2022 Nov 7. Proc Natl Acad Sci U S A. 2022. PMID: 36343260 Free PMC article.
-
Primary Carnitine Deficiency: Is Foetal Development Affected and Can Newborn Screening Be Improved?JIMD Rep. 2017;36:35-40. doi: 10.1007/8904_2016_30. Epub 2017 Jan 20. JIMD Rep. 2017. PMID: 28105570 Free PMC article.
-
Biochemical Markers for the Diagnosis of Mitochondrial Fatty Acid Oxidation Diseases.J Clin Med. 2021 Oct 22;10(21):4855. doi: 10.3390/jcm10214855. J Clin Med. 2021. PMID: 34768374 Free PMC article. Review.
References
-
- Rasmussen J., Nielsen O.W., Lund A.M., Kober L., Djurhuus H. Primary carnitine deficiency and pivalic acid exposure causing encephalopathy and fatal cardiac events. J. Inherit. Metab. Dis. 2013;36:35–41. - PubMed
-
- Rasmussen O.W. Nielsen, Janzen N., Duno M., Kober L., Steuerwald U., Lund A.M. Carnitine levels in 26,462 individuals from the nationwide screening program for primary carnitine deficiency in the Faroe Islands. J. Inherit. Metab. Dis. 2013;37:215–222. - PubMed
-
- Nezu J., Tamai I., Oku A., Ohashi R., Yabuuchi H., Hashimoto N., Nikaido H., Sai Y., Koizumi A., Shoji Y., Takada G., Matsuishi T., Yoshino M., Kato H., Ohura T., Tsujimoto G., Hayakawa J., Shimane M., Tsuji A. Primary systemic carnitine deficiency is caused by mutations in a gene encoding sodium ion-dependent carnitine transporter. Nat. Genet. 1999;21:91–94. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous