In Vitro Bone Cell Models: Impact of Fluid Shear Stress on Bone Formation
- PMID: 27896266
- PMCID: PMC5108781
- DOI: 10.3389/fbioe.2016.00087
In Vitro Bone Cell Models: Impact of Fluid Shear Stress on Bone Formation
Abstract
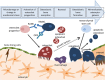
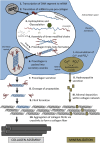
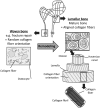
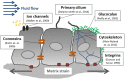
This review describes the role of bone cells and their surrounding matrix in maintaining bone strength through the process of bone remodeling. Subsequently, this work focusses on how bone formation is guided by mechanical forces and fluid shear stress in particular. It has been demonstrated that mechanical stimulation is an important regulator of bone metabolism. Shear stress generated by interstitial fluid flow in the lacunar-canalicular network influences maintenance and healing of bone tissue. Fluid flow is primarily caused by compressive loading of bone as a result of physical activity. Changes in loading, e.g., due to extended periods of bed rest or microgravity in space are associated with altered bone remodeling and formation in vivo. In vitro, it has been reported that bone cells respond to fluid shear stress by releasing osteogenic signaling factors, such as nitric oxide, and prostaglandins. This work focusses on the application of in vitro models to study the effects of fluid flow on bone cell signaling, collagen deposition, and matrix mineralization. Particular attention is given to in vitro set-ups, which allow long-term cell culture and the application of low fluid shear stress. In addition, this review explores what mechanisms influence the orientation of collagen fibers, which determine the anisotropic properties of bone. A better understanding of these mechanisms could facilitate the design of improved tissue-engineered bone implants or more effective bone disease models.
Keywords: bone remodeling; collagen orientation; fluid shear stress; osteoblast; osteocyte.
Figures

References
-
- Ajubi N. E., Klein-Nulend J., Alblas M. J., Burger E. H., Nijweide P. J. (1999). Signal transduction pathways involved in fluid flow-induced PGE2 production by cultured osteocytes. Am. J. Physiol. Endocrinol. Metab. 276, E171–E178. - PubMed
-
- Anderson H. C. (1995). Molecular biology of matrix vesicles. Clin. Orthop. Relat. Res. 314, 266–280. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

