A telephone-based intervention to promote physical activity during smoking cessation: a randomized controlled proof-of-concept study
- PMID: 27896797
- PMCID: PMC5526805
- DOI: 10.1007/s13142-016-0449-x
A telephone-based intervention to promote physical activity during smoking cessation: a randomized controlled proof-of-concept study
Abstract
Smoking and physical inactivity contribute to disproportionate disease burden among underserved adults. Telephone-based interventions (quitlines) are becoming the standard care for addressing smoking. There is increasing interest to determine whether quitlines can be utilized to administer interventions for other unhealthy behaviors. This study aims to examine the proof-of-concept and potential efficacy of a telephone-based behavioral counseling intervention to boost daily low-to-moderate physical activity among low-income, physically inactive smokers. Participants (N = 101) were randomized to receive 4 weeks of counseling prior to their smoking quit day that included either standard smoking cessation counseling (control) or the Step-up to Quit (SUTQ) intervention. SUTQ promoted daily walking to foster physical activity as a primary smoking urge management strategy and facilitate incremental increases in daily steps with the goal of achieving 7500 steps/day by the quit day in week 4. Exploratory structural equation modeling tested SUTQ effects on six measures of low-to-moderate physical activity (primary outcome) and smoking cue reactivity (secondary outcome) simultaneously in a single multivariate model with controlling variables. The sample was 51 % female and 77 % African-American, with a mean age of 42.1 years (SD = 10.9). Compared to the control condition, SUTQ intervention was associated with greater physical activity at week 4 (b = 0.51, z = 1.71, p = 0.08), with between-group differences sustained at follow-up. At week 4, the SUTQ group had higher 7-day mean steps/day (M = 7,207.25, SD = 4,276.03) than controls (M = 3,947.03, SD = 3,655.03) (t = 3.35; p < .01); and had more participants reach the >7500 steps/day goal (49% vs. 11 %, c2 = 10.78; p < .01), a difference that was sustained at 1-month follow-up (X2 = 9.04, p < .01) Effects of SUTQ treatment on cue reactivity were in the hypothesized direction but not significant (b = -0.29; z = -1.09, p = 0.27). To our knowledge, this is the first study to promote physical activity using telephone counseling in an underserved population of smokers known to have greater challenges with physical activity adoption and smoking cessation. The SUTQ approach suggests that integration of physical activity advice and support within the context of smoking cessation treatment has the potential to promote physical activity among smokers intending to quit.
Trial registration: ClinicalTrials.gov NCT02220465.
Keywords: Low-income smokers; Physical activity; Smoking cue reactivity; Telephone-based counseling.
Conflict of interest statement
Funding information
This study was funded by the American Heart Association (13CRP14560028). AHA is not involved in the study design, data collection, analysis or interpretation of data, and in the decision to submit the article for publication. Research reported in this publication was also supported by an Institutional Development Award (IDeA) Center of Biomedical Research Excellence from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM113125 (FP).
Statement on human rights
All procedures were performed in studies involving human participants were in accordance with the ethical standards of the institutional review board and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Statement on welfare of animals
This article does not contain any studies with animals performed by any of the authors.
Conflict of interests
None of the authors have any conflicts of interest associated with this manuscript.
Informed consent
Informed consent was obtained from all individual participants included in the study.
IRB approval
The study was approved by Temple University’s Institutional review Board (Project # 21109).
Figures
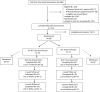
References
-
- Centers for Disease Control and Prevention. Best practices for comprehensive tobacco control programs—2014; 2014. http://www.cdc.gov/tobacco/stateandcommunity/best_practices/index.htm?so....
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

