The effect of craniokinesis on the middle ear of domestic chickens (Gallus gallus domesticus)
- PMID: 27896803
- PMCID: PMC5314387
- DOI: 10.1111/joa.12566
The effect of craniokinesis on the middle ear of domestic chickens (Gallus gallus domesticus)
Abstract
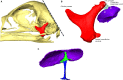
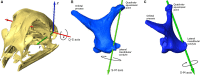
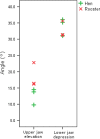
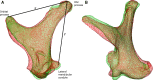
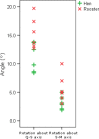
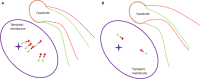
The avian middle ear differs from that of mammalians and contains a tympanic membrane, one ossicle (bony columella and cartilaginous extra-columella), some ligaments and one muscle. The rim of the eardrum (closing the middle ear cavity) is connected to the neurocranium and, by means of a broad ligament, to the otic process of the quadrate. Due to the limited number of components in the avian middle ear, the possibilities of attenuating the conduction of sound seem to be limited to activity of the stapedius muscle. We investigate to what extent craniokinesis may impact the components of the middle ear because of the connection of the eardrum to the movable quadrate. The quadrate is a part of the beak suspension and plays an important role in craniokinesis. Micro-computed tomography was used to visualize morphology and the effect of craniokinesis on the middle ear in the domestic chicken (Gallus gallus domesticus). Both hens and roosters are considered because of their difference in vocalization capacity. It is hypothesized that effects, if present, of craniokinesis on the middle ear will be greater in roosters because of their louder vocalization. Maximal lower jaw depression was comparable for hens and roosters (respectively 34.1 ± 2.6° and 32.7 ± 2.5°). There is no overlap in ranges of maximal upper jaw elevation between the sexes (respectively 12.7 ± 2.5° and 18.5 ± 3.8°). Frontal rotation about the transversal quadrato-squamosal, and inward rotation about the squamosal-mandibular axes of the quadrate were both considered to be greater in roosters (respectively 15.4 ± 2.8° and 11.1 ± 2.5°). These quadrate rotations did not affect the columellar position or orientation. In hens, an influence of the quadrate movements on the shape of the eardrum could not be detected either; however, craniokinesis caused slight stretching of the eardrum towards the caudal rim of the otic process of the quadrate. In roosters, an inward displacement of the conical tip of the tympanic membrane of 0.378 ± 0.21 mm, as a result of craniokinesis, was observed. This is linked to a flattening and slackening of the eardrum. These changes most likely go along with a deformation of the extra-columella. Generally, in birds, larger beak opening is related to the intensity of vocalization. The coupling between larger maximal upper jaw lifting in roosters and the slackening of the eardrum suggest the presence of a passive sound attenuation mechanism during self-vocalization.
Keywords: chicken; craniokinesis; micro-CT; middle ear; quadrate.
© 2016 Anatomical Society.
Figures



References
-
- Ades HW, Axelsson A, Baird I, et al. (2012) Auditory System: Anatomy Physiology (Ear). Berlin: Springer Science & Business Media.
-
- Bock WJ (1964) Kinetics of the avian skull. J Morphol 114, 1–41.
-
- Borg E, Counter SA, Rydqvist B (1979) Contraction properties and functional morphology of the avian stapedius muscle. Acta Otolaryngol 88, 20–26. - PubMed
-
- Borg E, Counter SA, Lännergren J (1982) Analysis of the avian middle ear muscle contraction by strain gauge and volume and impedance change measures. Comp Biochem Physiol A Comp Physiol 71, 619–621. - PubMed
-
- Bout RG, Zweers GA (2001) The role of cranial kinesis in birds. Comp Biochem Physiol A Mol Integr Physiol 131, 197–205. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

