A Rapid Cell Expansion Process for Production of Engineered Autologous CAR-T Cell Therapies
- PMID: 27897048
- PMCID: PMC6445175
- DOI: 10.1089/hgtb.2016.120
A Rapid Cell Expansion Process for Production of Engineered Autologous CAR-T Cell Therapies
Erratum in
-
Correction to: Hum Gene Ther Methods 2016;27:209-218.Hum Gene Ther Methods. 2017 Apr;28(2):100. doi: 10.1089/hgtb.2016.120.correx. Epub 2017 Feb 22. Hum Gene Ther Methods. 2017. PMID: 28394690 Free PMC article. No abstract available.
Abstract
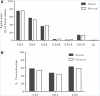
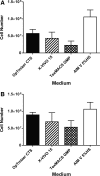
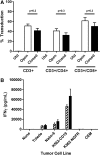
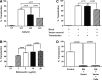
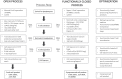
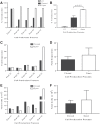
The treatment of B-cell malignancies by adoptive cell transfer (ACT) of anti-CD19 chimeric antigen receptor T cells (CD19 CAR-T) has proven to be a highly successful therapeutic modality in several clinical trials.1-6 The anti-CD19 CAR-T cell production method used to support initial trials relied on numerous manual, open process steps, human serum, and 10 days of cell culture to achieve a clinical dose.7 This approach limited the ability to support large multicenter clinical trials, as well as scale up for commercial cell production. Therefore, studies were completed to streamline and optimize the original National Cancer Institute production process by removing human serum from the process in order to minimize the risk of viral contamination, moving process steps from an open system to functionally closed system operations in order to minimize the risk of microbial contamination, and standardizing additional process steps in order to maximize process consistency. This study reports a procedure for generating CD19 CAR-T cells in 6 days, using a functionally closed manufacturing process and defined, serum-free medium. This method is able to produce CD19 CAR-T cells that are phenotypically and functionally indistinguishable from cells produced for clinical trials by the previously described production process.
Keywords: GMP; anti-CD19 CAR; closed system; cryopreservation; expansion; transduction.
Conflict of interest statement
M.B. has employment and equity ownership in Kite Pharma. O.P. is a former KITE Pharma employee with no outstanding financial interests. No competing financial interests exist for the remaining authors.
Figures
References
-
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2013. Bethedsda, MD: National Cancer Institute, 2016
-
- Kenkre VP, Smith SM. Management of relapsed diffuse large B-cell lymphoma. Curr Oncol Rep 2008;10:393–403 - PubMed
-
- Sehn LH, Fenske TS, Laport GG. Follicular lymphoma: prognostic factors, conventional therapies, and hematopoietic cell transplantation. Biol Blood Marrow Transplant 2012;18:S82–91 - PubMed
-
- Tedder TF, Isaacs CM. Isolation of cDNAs encoding the CD19 antigen of human and mouse B lymphocytes. A new member of the immunoglobulin superfamily. J Immunol 1989;143:712–717 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

