Design and development of novel antibacterial Ti-Ni-Cu shape memory alloys for biomedical application
- PMID: 27897182
- PMCID: PMC5126636
- DOI: 10.1038/srep37475
Design and development of novel antibacterial Ti-Ni-Cu shape memory alloys for biomedical application
Abstract
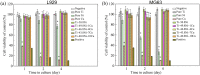
In the case of medical implants, foreign materials are preferential sites for bacterial adhesion and microbial contamination, which can lead to the development of prosthetic infections. Commercially biomedical TiNi shape memory alloys are the most commonly used materials for permanent implants in contact with bone and dental, and the prevention of infections of TiNi biomedical shape memory alloys in clinical cases is therefore a crucial challenge for orthopaedic and dental surgeons. In the present study, copper has been chosen as the alloying element for design and development novel ternary biomedical Ti‒Ni‒Cu shape memory alloys with antibacterial properties. The effects of copper alloying element on the microstructure, mechanical properties, corrosion behaviors, cytocompatibility and antibacterial properties of biomedical Ti‒Ni‒Cu shape memory alloys have been systematically investigated. The results demonstrated that Ti‒Ni‒Cu alloys have good mechanical properties, and remain the excellent shape memory effects after adding copper alloying element. The corrosion behaviors of Ti‒Ni‒Cu alloys are better than the commercial biomedical Ti‒50.8Ni alloys. The Ti‒Ni‒Cu alloys exhibit excellent antibacterial properties while maintaining the good cytocompatibility, which would further guarantee the potential application of Ti‒Ni‒Cu alloys as future biomedical implants and devices without inducing bacterial infections.
Figures







Similar articles
-
Antibacterial titanium surfaces for medical implants.Mater Sci Eng C Mater Biol Appl. 2016 Apr 1;61:965-78. doi: 10.1016/j.msec.2015.12.062. Epub 2015 Dec 31. Mater Sci Eng C Mater Biol Appl. 2016. PMID: 26838926 Review.
-
Length-dependent corrosion behavior, Ni2+ release, cytocompatibility, and antibacterial ability of Ni-Ti-O nanopores anodically grown on biomedical NiTi alloy.Mater Sci Eng C Mater Biol Appl. 2018 Aug 1;89:1-7. doi: 10.1016/j.msec.2018.03.018. Epub 2018 Mar 21. Mater Sci Eng C Mater Biol Appl. 2018. PMID: 29752078
-
Effect of the existing form of Cu element on the mechanical properties, bio-corrosion and antibacterial properties of Ti-Cu alloys for biomedical application.Mater Sci Eng C Mater Biol Appl. 2016 Dec 1;69:1210-21. doi: 10.1016/j.msec.2016.08.033. Epub 2016 Aug 13. Mater Sci Eng C Mater Biol Appl. 2016. PMID: 27612819
-
The antibacterial properties and biocompatibility of a Ti-Cu sintered alloy for biomedical application.Biomed Mater. 2014 Apr;9(2):025013. doi: 10.1088/1748-6041/9/2/025013. Epub 2014 Feb 24. Biomed Mater. 2014. PMID: 24565798
-
A new look at biomedical Ti-based shape memory alloys.Acta Biomater. 2012 May;8(5):1661-9. doi: 10.1016/j.actbio.2012.01.018. Epub 2012 Jan 28. Acta Biomater. 2012. PMID: 22326786 Review.
Cited by
-
Additively manufactured Ti-Ta-Cu alloys for the next-generation load-bearing implants.Int J Extrem Manuf. 2024 Feb 1;6(1):015503. doi: 10.1088/2631-7990/ad07e7. Epub 2023 Nov 17. Int J Extrem Manuf. 2024. PMID: 38021398 Free PMC article.
-
Actuating Bimorph Microstructures with Magnetron-Sputtered Ti-Ni-Cu Shape Memory Alloy Films.Nanomaterials (Basel). 2022 Nov 26;12(23):4207. doi: 10.3390/nano12234207. Nanomaterials (Basel). 2022. PMID: 36500830 Free PMC article.
-
Antibacterial and Antiviral Functional Materials: Chemistry and Biological Activity toward Tackling COVID-19-like Pandemics.ACS Pharmacol Transl Sci. 2020 Dec 29;4(1):8-54. doi: 10.1021/acsptsci.0c00174. eCollection 2021 Feb 12. ACS Pharmacol Transl Sci. 2020. PMID: 33615160 Free PMC article. Review.
-
Fabricated High-Strength, Low-Elastic Modulus Biomedical Ti-24Nb-4Zr-8Sn Alloy via Powder Metallurgy.Materials (Basel). 2023 May 19;16(10):3845. doi: 10.3390/ma16103845. Materials (Basel). 2023. PMID: 37241471 Free PMC article.
-
Multifunctional coatings of nickel-titanium implant toward promote osseointegration after operation of bone tumor and clinical application: a review.Front Bioeng Biotechnol. 2024 Feb 20;12:1325707. doi: 10.3389/fbioe.2024.1325707. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 38444648 Free PMC article. Review.
References
-
- El Feninat F., Laroche G., Fiset M. & Mantovani D. Shape memory materials for biomedical applications. Adv Eng Mater 4, 91–104 (2002).
-
- Biesiekierski A., Wang J., Gepreel M. A. H. & Wen C. A new look at biomedical Ti-based shape memory alloys. Acta Biomater 8, 1661–1669 (2012). - PubMed
-
- Zhao T. T., Li Y., Wei S. B. & Xiang Y. Research Progress on Surface Modification of Biomedical TiNi Shape Memory Alloys. Rare Metal Mat Eng 39, 320–323 (2010).
-
- Chu C. L. et al.. Surface structure and biomedical properties of chemically polished and electropolished NiTi shape memory alloys. Mat Sci Eng C-Bio S 28, 1430–1434 (2008).
-
- Ferraris S. & Spriano S. Antibacterial titanium surfaces for medical implants. Mat Sci Eng C-Mater 61, 965–978 (2016). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

