High-Entropy Metal Diborides: A New Class of High-Entropy Materials and a New Type of Ultrahigh Temperature Ceramics
- PMID: 27897255
- PMCID: PMC5126569
- DOI: 10.1038/srep37946
High-Entropy Metal Diborides: A New Class of High-Entropy Materials and a New Type of Ultrahigh Temperature Ceramics
Abstract
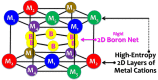
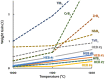
Seven equimolar, five-component, metal diborides were fabricated via high-energy ball milling and spark plasma sintering. Six of them, including (Hf0.2Zr0.2Ta0.2Nb0.2Ti0.2)B2, (Hf0.2Zr0.2Ta0.2Mo0.2Ti0.2)B2, (Hf0.2Zr0.2Mo0.2Nb0.2Ti0.2)B2, (Hf0.2Mo0.2Ta0.2Nb0.2Ti0.2)B2, (Mo0.2Zr0.2Ta0.2Nb0.2Ti0.2)B2, and (Hf0.2Zr0.2Ta0.2Cr0.2Ti0.2)B2, possess virtually one solid-solution boride phase of the hexagonal AlB2 structure. Revised Hume-Rothery size-difference factors are used to rationalize the formation of high-entropy solid solutions in these metal diborides. Greater than 92% of the theoretical densities have been generally achieved with largely uniform compositions from nanoscale to microscale. Aberration-corrected scanning transmission electron microscopy (AC STEM), with high-angle annular dark-field and annular bright-field (HAADF and ABF) imaging and nanoscale compositional mapping, has been conducted to confirm the formation of 2-D high-entropy metal layers, separated by rigid 2-D boron nets, without any detectable layered segregation along the c-axis. These materials represent a new type of ultra-high temperature ceramics (UHTCs) as well as a new class of high-entropy materials, which not only exemplify the first high-entropy non-oxide ceramics (borides) fabricated but also possess a unique non-cubic (hexagonal) and layered (quasi-2D) high-entropy crystal structure that markedly differs from all those reported in prior studies. Initial property assessments show that both the hardness and the oxidation resistance of these high-entropy metal diborides are generally higher/better than the average performances of five individual metal diborides made by identical fabrication processing.
Figures

 zone axis of hexagonal structure. (0001) and
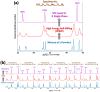
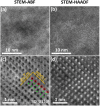
zone axis of hexagonal structure. (0001) and  planes are indexed in (c). The red circles highlight the columns of transition metal atoms (Hf, Zr, Ta, Mo and Ti). The green dots indicate the B atoms. Additional STEM images from different regions and a different specimen are documented in the Supplementary Figs S8–S10; a further digital analysis of HAADF and ABF images in Supplementary Fig. S11 shows that the standard variations in the (0001) lattice spacings are only ~0.6% or ~0.02 Å, indicating homogenous mixing of five metal atoms (Hf, Zr, Ta, Mo and Ti) within the 2-D metal layers in (0001) planes.
planes are indexed in (c). The red circles highlight the columns of transition metal atoms (Hf, Zr, Ta, Mo and Ti). The green dots indicate the B atoms. Additional STEM images from different regions and a different specimen are documented in the Supplementary Figs S8–S10; a further digital analysis of HAADF and ABF images in Supplementary Fig. S11 shows that the standard variations in the (0001) lattice spacings are only ~0.6% or ~0.02 Å, indicating homogenous mixing of five metal atoms (Hf, Zr, Ta, Mo and Ti) within the 2-D metal layers in (0001) planes.
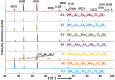
 zone axis, showing no significant layer-to-layer variations of metal composition in different basal (0001) planes. Additional EDX compositional maps obtained from a different region are documented in the Supplementary Fig. S12.
zone axis, showing no significant layer-to-layer variations of metal composition in different basal (0001) planes. Additional EDX compositional maps obtained from a different region are documented in the Supplementary Fig. S12.
References
-
- Zhang Y., Zuo T. T., Tang Z., Gao M. C., Dahmen K. A., Liaw P. K. & Lu Z. P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 61, 1–93 (2014).
-
- Tsai M.-H. & Yeh J.-W. High-Entropy Alloys: A Critical Review. Mater. Res. Lett. 2, 107–123 (2014).
-
- Gludovatz B., Hohenwarter A., Catoor D., Chang E. H., George E. P. & Ritchie R. O. A fracture-resistant high-entropy alloy for cryogenic applications. Science 345, 1153–1158 (2014). - PubMed
-
- Poulia A., Georgatis E., Lekatou A. & Karantzalis A. Microstructure and wear behavior of a refractory high entropy alloy. Int. J. Refractory Met. Hard Mater. 57, 50–63 (2016).
-
- Fazakas E., Zadorozhnyy V., Varga L., Inoue A., Louzguine-Luzgin D., Tian F. & Vitos L. Experimental and theoretical study of Ti20Zr20Hf20Nb20X20 (X = V or Cr) refractory high-entropy alloys. Int. J. Refractory Met. Hard Mater. 47, 131–138 (2014).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

