BCl3 -Induced Annulative Oxo- and Thioboration for the Formation of C3-Borylated Benzofurans and Benzothiophenes
- PMID: 27897368
- PMCID: PMC5396270
- DOI: 10.1002/anie.201610014
BCl3 -Induced Annulative Oxo- and Thioboration for the Formation of C3-Borylated Benzofurans and Benzothiophenes
Abstract
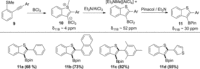
BCl3 -induced borylative cyclization of aryl-alkynes possessing ortho-EMe (E=S, O) groups represents a simple, metal-free method for the formation of C3-borylated benzothiophenes and benzofurans. The dichloro(heteroaryl)borane primary products can be protected to form synthetically ubiquitous pinacol boronate esters or used in situ in Suzuki-Miyaura cross couplings to generate 2,3-disubstituted heteroarenes from simple alkyne precursors in one pot. In a number of cases alkyne trans-haloboration occurs alongside, or instead of, borylative cyclization and the factors controlling the reaction outcome are determined.
Keywords: annulation; borylation; cross coupling; electrophilic cyclization; organoboranes.
© 2017 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures







References
-
- 2,3-substituted benzofurans and benzothiophenes are privileged structures e.g., in desketoraloxifene:
-
- Radadiya A., Shah A., Eur. J. Med. Chem. 2015, 97, 356; - PubMed
-
- For an example of a materials application see: Tsuji H., Mitsui C., Ilies L., Sato Y., Nakamura E., J. Am. Chem. Soc. 2007, 129, 11902. - PubMed
-
- None
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

