Lipid modulation of skeletal muscle mass and function
- PMID: 27897400
- PMCID: PMC5377414
- DOI: 10.1002/jcsm.12144
Lipid modulation of skeletal muscle mass and function
Abstract
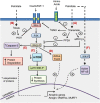
Loss of skeletal muscle mass is a characteristic feature of various pathologies including cancer, diabetes, and obesity, as well as being a general feature of ageing. However, the processes underlying its pathogenesis are not fully understood and may involve multiple factors. Importantly, there is growing evidence which supports a role for fatty acids and their derived lipid intermediates in the regulation of skeletal muscle mass and function. In this review, we discuss evidence pertaining to those pathways which are involved in the reduction, increase and/or preservation of skeletal muscle mass by such lipids under various pathological conditions, and highlight studies investigating how these processes may be influenced by dietary supplementation as well as genetic and/or pharmacological intervention.
Keywords: Atrophy; Catabolism; Fatty acid; Lipid; Obesity; Skeletal muscle; mTOR.
© 2016 The Authors. Journal of Cachexia, Sarcopenia and Muscle published by John Wiley & Sons Ltd on behalf of the Society on Sarcopenia, Cachexia and Wasting Disorders.
Figures

Similar articles
-
Effects of supplementation with branched-chain amino acids to low-protein diets on expression of genes related to lipid metabolism in skeletal muscle of growing pigs.Amino Acids. 2016 Sep;48(9):2131-44. doi: 10.1007/s00726-016-2223-2. Epub 2016 May 7. Amino Acids. 2016. PMID: 27156063
-
The impact of ageing, physical activity, and pre-frailty on skeletal muscle phenotype, mitochondrial content, and intramyocellular lipids in men.J Cachexia Sarcopenia Muscle. 2017 Apr;8(2):213-228. doi: 10.1002/jcsm.12139. Epub 2016 Sep 2. J Cachexia Sarcopenia Muscle. 2017. PMID: 27897402 Free PMC article.
-
Diminished anabolic signaling response to insulin induced by intramuscular lipid accumulation is associated with inflammation in aging but not obesity.Am J Physiol Regul Integr Comp Physiol. 2016 Apr 1;310(7):R561-9. doi: 10.1152/ajpregu.00198.2015. Epub 2016 Jan 13. Am J Physiol Regul Integr Comp Physiol. 2016. PMID: 26764052 Free PMC article.
-
Free fatty acids and skeletal muscle insulin resistance.Prog Mol Biol Transl Sci. 2014;121:267-92. doi: 10.1016/B978-0-12-800101-1.00008-9. Prog Mol Biol Transl Sci. 2014. PMID: 24373240 Review.
-
Impaired protein metabolism: interlinks between obesity, insulin resistance and inflammation.Obes Rev. 2012 Dec;13 Suppl 2:51-7. doi: 10.1111/j.1467-789X.2012.01037.x. Obes Rev. 2012. PMID: 23107259 Review.
Cited by
-
Lipid metabolites and sarcopenia-related traits: a Mendelian randomization study.Diabetol Metab Syndr. 2024 Sep 16;16(1):231. doi: 10.1186/s13098-024-01465-y. Diabetol Metab Syndr. 2024. PMID: 39285470 Free PMC article.
-
Defect in cytosolic Neu2 sialidase abrogates lipid metabolism and impairs muscle function in vivo.Sci Rep. 2022 Feb 25;12(1):3216. doi: 10.1038/s41598-022-07033-6. Sci Rep. 2022. PMID: 35217678 Free PMC article.
-
CT-based assessment of sarcopenia for differentiating wild-type from mutant-type gastrointestinal stromal tumor.Sci Rep. 2023 Feb 24;13(1):3216. doi: 10.1038/s41598-022-27213-8. Sci Rep. 2023. PMID: 36828845 Free PMC article.
-
Association of Adductor Pollicis Muscle Thickness and Handgrip Strength with nutritional status in cancer patients.PLoS One. 2019 Aug 2;14(8):e0220334. doi: 10.1371/journal.pone.0220334. eCollection 2019. PLoS One. 2019. PMID: 31374093 Free PMC article.
-
Polyunsaturated Fatty Acids Level and Bone Mineral Density: A Two-Sample Mendelian Randomization Study.Front Endocrinol (Lausanne). 2022 Jul 8;13:858851. doi: 10.3389/fendo.2022.858851. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35872990 Free PMC article.
References
-
- Sullivan MJ, Green HJ, Cobb FR. Skeletal muscle biochemistry and histology in ambulatory patients with long‐term heart failure. Circulation 1990;81:518–27. - PubMed
-
- Vescovo G, Serafini F, Facchin L, Tenderini P, Carraro U, Dalla Libera L, et al. Specific changes in skeletal muscle myosin heavy chain composition in cardiac failure: differences compared with disuse atrophy as assessed on microbiopsies by high resolution electrophoresis. Heart (Br Cardiac Soc) 1996;76:337–43. - PMC - PubMed
-
- Grady RM, Teng H, Nichol MC, Cunningham JC, Wilkinson RS, Sanes JR. Skeletal and cardiac myopathies in mice lacking utrophin and dystrophin: a model for Duchenne muscular dystrophy. Cell 1997;90:729–38. - PubMed
-
- Whitehouse AS, Smith HJ, Drake JL, Tisdale MJ. Mechanism of attenuation of skeletal muscle protein catabolism in cancer cachexia by eicosapentaenoic acid. Cancer Res 2001;61:3604–9. - PubMed
-
- Glass DJ. Signalling pathways that mediate skeletal muscle hypertrophy and atrophy. Nat Cell Biol 2003;5:87–90. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

