The Antiproliferative Effect of Chakasaponins I and II, Floratheasaponin A, and Epigallocatechin 3-O-Gallate Isolated from Camellia sinensis on Human Digestive Tract Carcinoma Cell Lines
- PMID: 27898032
- PMCID: PMC5187779
- DOI: 10.3390/ijms17121979
The Antiproliferative Effect of Chakasaponins I and II, Floratheasaponin A, and Epigallocatechin 3-O-Gallate Isolated from Camellia sinensis on Human Digestive Tract Carcinoma Cell Lines
Abstract
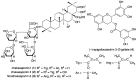
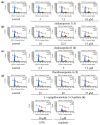
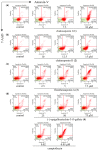
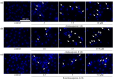
Acylated oleanane-type triterpene saponins, namely chakasaponins I (1) and II (2), floratheasaponin A (3), and their analogs, together with catechins-including (-)-epigallocatechin 3-O-gallate (4), flavonoids, and caffeine-have been isolated as characteristic functional constituents from the extracts of "tea flower", the flower buds of Camellia sinensis (Theaceae), which have common components with that of the leaf part. These isolates exhibited antiproliferative activities against human digestive tract carcinoma HSC-2, HSC-4, MKN-45, and Caco-2 cells. The antiproliferative activities of the saponins (1-3, IC50 = 4.4-14.1, 6.2-18.2, 4.5-17.3, and 19.3-40.6 µM, respectively) were more potent than those of catechins, flavonoids, and caffeine. To characterize the mechanisms of action of principal saponin constituents 1-3, a flow cytometric analysis using annexin-V/7-aminoactinomycin D (7-AAD) double staining in HSC-2 cells was performed. The percentage of apoptotic cells increased in a concentration-dependent manner. DNA fragmentation and caspase-3/7 activation were also detected after 48 h. These results suggested that antiproliferative activities of 1-3 induce apoptotic cell death via activation of caspase-3/7.
Keywords: (–)-epigallocatechin 3-O-gallate; Camellia sinensis; anti-proliferative activity; apoptosis; chakasaponin; floratheasaponin; tea flower.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

