RNA Contaminates Glycosaminoglycans Extracted from Cells and Tissues
- PMID: 27898729
- PMCID: PMC5127559
- DOI: 10.1371/journal.pone.0167336
RNA Contaminates Glycosaminoglycans Extracted from Cells and Tissues
Abstract
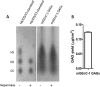
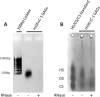
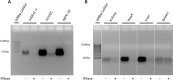
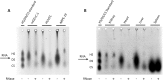
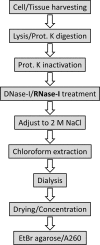
Glycosaminoglycans (GAGs) are linear negatively charged polysaccharides and important components of extracellular matrices and cell surface glycan layers such as the endothelial glycocalyx. The GAG family includes sulfated heparin, heparan sulfate (HS), dermatan sulfate (DS), chondroitin sulfate (CS), keratan sulfate, and non-sulfated hyaluronan. Because relative expression of GAGs is dependent on cell-type and niche, isolating GAGs from cell cultures and tissues may provide insight into cell- and tissue-specific GAG structure and functions. In our objective to obtain structural information about the GAGs expressed on a specialized mouse glomerular endothelial cell culture (mGEnC-1) we adapted a recently published GAG isolation protocol, based on cell lysis, proteinase K and DNase I digestion. Analysis of the GAGs contributing to the mGEnC-1 glycocalyx indicated a large HS and a minor CS content on barium acetate gel. However, isolated GAGs appeared resistant to enzymatic digestion by heparinases. We found that these GAG extracts were heavily contaminated with RNA, which co-migrated with HS in barium acetate gel electrophoresis and interfered with 1,9-dimethylmethylene blue (DMMB) assays, resulting in an overestimation of GAG yields. We hypothesized that RNA may be contaminating GAG extracts from other cell cultures and possibly tissue, and therefore investigated potential RNA contaminations in GAG extracts from two additional cell lines, human umbilical vein endothelial cells and retinal pigmental epithelial cells, and mouse kidney, liver, spleen and heart tissue. GAG extracts from all examined cell lines and tissues contained varying amounts of contaminating RNA, which interfered with GAG quantification using DMMB assays and characterization of GAGs by barium acetate gel electrophoresis. We therefore recommend routinely evaluating the RNA content of GAG extracts and propose a robust protocol for GAG isolation that includes an RNA digestion step.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
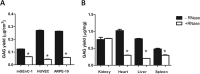
Similar articles
-
A biological guide to glycosaminoglycans: current perspectives and pending questions.FEBS J. 2024 Aug;291(15):3331-3366. doi: 10.1111/febs.17107. Epub 2024 Mar 18. FEBS J. 2024. PMID: 38500384 Review.
-
Synthesis and secretion of glycosaminoglycans in cultured retinal pigment epithelium.Invest Ophthalmol Vis Sci. 1987 Apr;28(4):618-27. Invest Ophthalmol Vis Sci. 1987. PMID: 3104227
-
Implementation of infrared and Raman modalities for glycosaminoglycan characterization in complex systems.Glycoconj J. 2017 Jun;34(3):309-323. doi: 10.1007/s10719-016-9743-6. Epub 2016 Dec 7. Glycoconj J. 2017. PMID: 27928742 Free PMC article. Review.
-
Benign hyperplasia of the human prostate is associated with tissue enrichment in chondroitin sulphate of wide size distribution.Prostate. 2000 Jul 1;44(2):104-10. doi: 10.1002/1097-0045(20000701)44:2<104::aid-pros2>3.0.co;2-6. Prostate. 2000. PMID: 10881019
-
Examination of corneal proteoglycans and glycosaminoglycans by rotary shadowing and electron microscopy.Int J Biol Macromol. 1990 Jun;12(3):180-4. doi: 10.1016/0141-8130(90)90029-a. Int J Biol Macromol. 1990. PMID: 2125466
Cited by
-
High-molecular weight hyaluronan attenuates tubulointerstitial scarring in kidney injury.JCI Insight. 2020 Jun 18;5(12):e136345. doi: 10.1172/jci.insight.136345. JCI Insight. 2020. PMID: 32396531 Free PMC article.
-
Identification of a S. aureus virulence factor by activity-based protein profiling (ABPP).Nat Chem Biol. 2018 Jun;14(6):609-617. doi: 10.1038/s41589-018-0060-1. Epub 2018 May 16. Nat Chem Biol. 2018. PMID: 29769740 Free PMC article.
-
Differential binding of chemokines CXCL1, CXCL2 and CCL2 to mouse glomerular endothelial cells reveals specificity for distinct heparan sulfate domains.PLoS One. 2018 Sep 24;13(9):e0201560. doi: 10.1371/journal.pone.0201560. eCollection 2018. PLoS One. 2018. PMID: 30248108 Free PMC article.
-
Glomerular endothelial glycocalyx-derived heparan sulfate inhibits glomerular leukocyte influx and attenuates experimental glomerulonephritis.Front Mol Biosci. 2023 Jun 1;10:1177560. doi: 10.3389/fmolb.2023.1177560. eCollection 2023. Front Mol Biosci. 2023. PMID: 37325479 Free PMC article.
-
Selective Binding of Heparin/Heparan Sulfate Oligosaccharides to Factor H and Factor H-Related Proteins: Therapeutic Potential for C3 Glomerulopathies.Front Immunol. 2021 Aug 18;12:676662. doi: 10.3389/fimmu.2021.676662. eCollection 2021. Front Immunol. 2021. PMID: 34489931 Free PMC article.
References
-
- Gotte M. Syndecans in inflammation. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2003;17(6):575–91. Epub 2003/04/01. - PubMed
-
- Koenig A, Norgard-Sumnicht K, Linhardt R, Varki A. Differential interactions of heparin and heparan sulfate glycosaminoglycans with the selectins. Implications for the use of unfractionated and low molecular weight heparins as therapeutic agents. The Journal of clinical investigation. 1998;101(4):877–89. Epub 1998/03/21. PubMed Central PMCID: PMC508636. 10.1172/JCI1509 - DOI - PMC - PubMed
-
- Coombe DR, Watt SM, Parish CR. Mac-1 (CD11b/CD18) and CD45 mediate the adhesion of hematopoietic progenitor cells to stromal cell elements via recognition of stromal heparan sulfate. Blood. 1994;84(3):739–52. Epub 1994/08/01. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

