Kluyveromyces marxianus and Saccharomyces boulardii Induce Distinct Levels of Dendritic Cell Cytokine Secretion and Significantly Different T Cell Responses In Vitro
- PMID: 27898740
- PMCID: PMC5127564
- DOI: 10.1371/journal.pone.0167410
Kluyveromyces marxianus and Saccharomyces boulardii Induce Distinct Levels of Dendritic Cell Cytokine Secretion and Significantly Different T Cell Responses In Vitro
Abstract
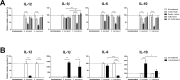
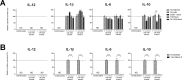
Interactions between members of the intestinal microbiota and the mucosal immune system can significantly impact human health, and in this context, fungi and food-related yeasts are known to influence intestinal inflammation through direct interactions with specialized immune cells in vivo. The aim of the present study was to characterize the immune modulating properties of the food-related yeast Kluyveromyces marxianus in terms of adaptive immune responses indicating inflammation versus tolerance and to explore the mechanisms behind the observed responses. Benchmarking against a Saccharomyces boulardii strain with probiotic effects documented in clinical trials, we evaluated the ability of K. marxianus to modulate human dendritic cell (DC) function in vitro. Further, we assessed yeast induced DC modulation of naive T cells toward effector responses dominated by secretion of IFNγ and IL-17 versus induction of a Treg response characterized by robust IL-10 secretion. In addition, we blocked relevant DC surface receptors and investigated the stimulating properties of β-glucan containing yeast cell wall extracts. K. marxianus and S. boulardii induced distinct levels of DC cytokine secretion, primarily driven by Dectin-1 recognition of β-glucan components in their cell walls. Upon co-incubation of yeast exposed DCs and naive T cells, S. boulardii induced a potent IFNγ response indicating TH1 mobilization. In contrast, K. marxianus induced a response dominated by Foxp3+ Treg cells, a characteristic that may benefit human health in conditions characterized by excessive inflammation and positions K. marxianus as a strong candidate for further development as a novel yeast probiotic.
Conflict of interest statement
I have read the journal’s policy and have the following conflicts to declare. IMS and AB are employees of Chr. Hansen A/S, a manufacturer of probiotic products, and the described work was carried out at Chr. Hansen A/S facilities in Hørsholm, Denmark. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures



Similar articles
-
Yeast modulation of human dendritic cell cytokine secretion: an in vitro study.PLoS One. 2014 May 9;9(5):e96595. doi: 10.1371/journal.pone.0096595. eCollection 2014. PLoS One. 2014. PMID: 24816850 Free PMC article.
-
Non-Saccharomyces yeasts protect against epithelial cell barrier disruption induced by Salmonella enterica subsp. enterica serovar Typhimurium.Lett Appl Microbiol. 2015 Nov;61(5):491-7. doi: 10.1111/lam.12481. Epub 2015 Sep 22. Lett Appl Microbiol. 2015. PMID: 26280244
-
Cancer Chemopreventive, Antiproliferative, and Superoxide Anion Scavenging Properties of Kluyveromyces marxianus and Saccharomyces cerevisiae var. boulardii Cell Wall Components.Nutr Cancer. 2018 Jan;70(1):83-96. doi: 10.1080/01635581.2018.1380204. Epub 2017 Nov 16. Nutr Cancer. 2018. PMID: 29144773
-
Yeasts as probiotics: Mechanisms, outcomes, and future potential.Fungal Genet Biol. 2020 Apr;137:103333. doi: 10.1016/j.fgb.2020.103333. Epub 2020 Jan 7. Fungal Genet Biol. 2020. PMID: 31923554 Review.
-
The effects of β-glucans on dendritic cells and implications for cancer therapy.Anticancer Agents Med Chem. 2013 Jun;13(5):689-98. doi: 10.2174/1871520611313050003. Anticancer Agents Med Chem. 2013. PMID: 23092290 Review.
Cited by
-
Emerging relevance of cell wall components from non-conventional yeasts as functional ingredients for the food and feed industry.Curr Res Food Sci. 2023 Sep 30;7:100603. doi: 10.1016/j.crfs.2023.100603. eCollection 2023. Curr Res Food Sci. 2023. PMID: 37840697 Free PMC article. Review.
-
The Interaction of Human Pathogenic Fungi With C-Type Lectin Receptors.Front Immunol. 2018 Jun 4;9:1261. doi: 10.3389/fimmu.2018.01261. eCollection 2018. Front Immunol. 2018. PMID: 29915598 Free PMC article. Review.
-
Cutting Edge: Probiotics and Fecal Microbiota Transplantation in Immunomodulation.J Immunol Res. 2019 Apr 16;2019:1603758. doi: 10.1155/2019/1603758. eCollection 2019. J Immunol Res. 2019. PMID: 31143780 Free PMC article. Review.
-
Effect of yeast probiotic Saccharomyces boulardii cell wall extract on Aspergillus fumigatus allergenicity in A549 cells.Curr Med Mycol. 2023 Dec;9(4):1-8. doi: 10.22034/cmm.2024.345134.1463. Curr Med Mycol. 2023. PMID: 38983617 Free PMC article.
-
Investigating the Efficacy of Saccharomyces boulardii in Metabolic Syndrome Treatment: A Narrative Review of What Is Known So Far.Int J Mol Sci. 2023 Jul 27;24(15):12015. doi: 10.3390/ijms241512015. Int J Mol Sci. 2023. PMID: 37569390 Free PMC article. Review.
References
-
- van der Aa Kuhle A, Jespersen L. The taxonomic position of Saccharomyces boulardii as evaluated by sequence analysis of the D1/D2 domain of 26S rDNA, the ITS1-5.8S rDNA-ITS2 region and the mitochondrial cytochrome-c oxidase II gene. Syst Appl Microbiol. 2003;26(4):564–71. 10.1078/072320203770865873 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

