Drug interventions for the treatment of obesity in children and adolescents
- PMID: 27899001
- PMCID: PMC6472619
- DOI: 10.1002/14651858.CD012436
Drug interventions for the treatment of obesity in children and adolescents
Abstract
Background: Child and adolescent obesity has increased globally, and can be associated with significant short- and long-term health consequences.
Objectives: To assess the efficacy of drug interventions for the treatment of obesity in children and adolescents.
Search methods: We searched CENTRAL, MEDLINE, Embase, PubMed (subsets not available on Ovid), LILACS as well as the trial registers ICTRP (WHO) and ClinicalTrials.gov. Searches were undertaken from inception to March 2016. We checked references and applied no language restrictions.
Selection criteria: We selected randomised controlled trials (RCTs) of pharmacological interventions for treating obesity (licensed and unlicensed for this indication) in children and adolescents (mean age under 18 years) with or without support of family members, with a minimum of three months' pharmacological intervention and six months' follow-up from baseline. We excluded interventions that specifically dealt with the treatment of eating disorders or type 2 diabetes, or included participants with a secondary or syndromic cause of obesity. In addition, we excluded trials which included growth hormone therapies and pregnant participants.
Data collection and analysis: Two review authors independently assessed trial quality and extracted data following standard Cochrane methodology. Where necessary we contacted authors for additional information.
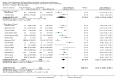
Main results: We included 21 trials and identified eight ongoing trials. The included trials evaluated metformin (11 trials), sibutramine (six trials), orlistat (four trials), and one trial arm investigated the combination of metformin and fluoxetine. The ongoing trials evaluated metformin (four trials), topiramate (two trials) and exenatide (two trials). A total of 2484 people participated in the included trials, 1478 participants were randomised to drug intervention and 904 to comparator groups (91 participants took part in two cross-over trials; 11 participants not specified). Eighteen trials used a placebo in the comparator group. Two trials had a cross-over design while the remaining 19 trials were parallel RCTs. The length of the intervention period ranged from 12 weeks to 48 weeks, and the length of follow-up from baseline ranged from six months to 100 weeks.Trials generally had a low risk of bias for random sequence generation, allocation concealment and blinding (participants, personnel and assessors) for subjective and objective outcomes. We judged approximately half of the trials as having a high risk of bias in one or more domain such as selective reporting.The primary outcomes of this review were change in body mass index (BMI), change in weight and adverse events. All 21 trials measured these outcomes. The secondary outcomes were health-related quality of life (only one trial reported results showing no marked differences; very low certainty evidence), body fat distribution (measured in 18 trials), behaviour change (measured in six trials), participants' views of the intervention (not reported), morbidity associated with the intervention (measured in one orlistat trial only reporting more new gallstones following the intervention; very low certainty evidence), all-cause mortality (one suicide in the orlistat intervention group; low certainty evidence) and socioeconomic effects (not reported).Intervention versus comparator for mean difference (MD) in BMI change was -1.3 kg/m2 (95% confidence interval (CI) -1.9 to -0.8; P < 0.00001; 16 trials; 1884 participants; low certainty evidence). When split by drug type, sibutramine, metformin and orlistat all showed reductions in BMI in favour of the intervention.Intervention versus comparator for change in weight showed a MD of -3.9 kg (95% CI -5.9 to -1.9; P < 0.00001; 11 trials; 1180 participants; low certainty evidence). As with BMI, when the trials were split by drug type, sibutramine, metformin and orlistat all showed reductions in weight in favour of the intervention.Five trials reported serious adverse events: 24/878 (2.7%) participants in the intervention groups versus 8/469 (1.7%) participants in the comparator groups (risk ratio (RR) 1.43, 95% CI 0.63 to 3.25; 1347 participants; low certainty evidence). A total 52/1043 (5.0%) participants in the intervention groups versus 17/621 (2.7%) in the comparator groups discontinued the trial because of adverse events (RR 1.45, 95% CI 0.83 to 2.52; 10 trials; 1664 participants; low certainty evidence). The most common adverse events in orlistat and metformin trials were gastrointestinal (such as diarrhoea, mild abdominal pain or discomfort, fatty stools). The most frequent adverse events in sibutramine trials included tachycardia, constipation and hypertension. The single fluoxetine trial reported dry mouth and loose stools. No trial investigated drug treatment for overweight children.
Authors' conclusions: This systematic review is part of a series of associated Cochrane reviews on interventions for obese children and adolescents and has shown that pharmacological interventions (metformin, sibutramine, orlistat and fluoxetine) may have small effects in reduction in BMI and bodyweight in obese children and adolescents. However, many of these drugs are not licensed for the treatment of obesity in children and adolescents, or have been withdrawn. Trials were generally of low quality with many having a short or no post-intervention follow-up period and high dropout rates (overall dropout of 25%). Future research should focus on conducting trials with sufficient power and long-term follow-up, to ensure the long-term effects of any pharmacological intervention are comprehensively assessed. Adverse events should be reported in a more standardised manner specifying amongst other things the number of participants experiencing at least one adverse event. The requirement of regulatory authorities (US Food and Drug Administration and European Medicines Agency) for trials of all new medications to be used in children and adolescents should drive an increase in the number of high quality trials.
Conflict of interest statement
EM: none known. GA: none known. BR: none known. MIM: none known. LB: none known. NF: has provided medical consultancy to several pharmaceutical companies developing and marketing (outside of the UK at present) treatments for obesity. Since March 2016 he is employed by Novo Nordisk, Denmark in Global Medical Affairs. The review was submitted for publication in November 2015, pre‐dating an offer of employment by Novo Nordisk A/S made in December 2015.
Figures

















References
References to studies included in this review
Atabek 2008 {published data only}
-
- Atabek ME, Pirgon O. Use of metformin in obese adolescents with hyperinsulinemia: a 6‐month, randomized, double‐blind, placebo‐controlled clinical trial. Journal of Pediatric Endocrinology and Metabolism 2008;21(4):339‐48. - PubMed
Berkowitz 2003 {published data only}
-
- Berkowitz RI, Wadden TA, Tershakovec AM, Cronquist JL. Behavior therapy and sibutramine for the treatment of adolescent obesity: a randomized controlled trial. JAMA 2003;289(14):1805‐12. - PubMed
-
- Budd GM, Hayman LL, Crump E, Pollydore C, Hawley K, Cronquist JL, et al. Weight loss in obese African American and Caucasian adolescents. Journal of Cardiovascular Nursing 2007;22(4):288‐96. - PubMed
Berkowitz 2006 {published data only}
-
- Berkowitz RI, Fujioka K, Daniels SR, Hoppin AG, Owen S, Perry AC, et al. Effects of sibutramine treatment in obese adolescents: a randomized trial. Annals of Internal Medicine 2006;145(2):81‐90. - PubMed
Chanoine 2005 {published and unpublished data}
-
- Chanoine J, Hampl S, Jensen C, Boldrin M, Hauptman J. Effect of orlistat on weight and body composition in obese adolescents: a randomized controlled trial. JAMA 2005;293(23):2873‐83. - PubMed
Clarson 2009 {published data only}
-
- Clarson CL, Mahmud FH, Baker JE, Clark HE, Mckay WM, Schauteet VD, et al. Metformin in combination with structured lifestyle intervention improved body mass index in obese adolescents, but did not improve insulin resistance. Endocrine 2009;36(1):141‐6. [DOI: 10.1007/s12020-009-9196-9] - DOI - PubMed
Franco 2014 {published data only}
-
- Franco RR, Cominato L, Damiani D. The effect of sibutramine on weight loss in obese adolescents [O efeito da sibutramina na perda de peso de adolescentes obesos]. Arquivos Brasileiros de Endocrinologia e Metabologia 2014;58:243‐50. - PubMed
Freemark 2001 {published and unpublished data}
-
- Freemark M, Bursey D. The effects of metformin on body mass index and glucose tolerance in obese adolescents with fasting hyperinsulinemia and a family history of type 2 diabetes. Pediatrics 2001;107(4):e55. - PubMed
García‐Morales 2006 {published data only}
-
- García‐Morales LM, Berber A, Macias‐Lara CC, Lucio‐Ortiz C, Del‐Rio‐Navarro BE, Dorantes‐Alvárez LM. Use of sibutramine in obese Mexican adolescents: a 6‐month, randomized, double‐blind, placebo‐controlled, parallel‐group trial. Clinical Therapeutics 2006;28(5):770‐82. [DOI: 10.1016/j.clinthera.2006.05.008] - DOI - PubMed
Godoy‐Matos 2005 {published data only}
-
- Correa LL, Platt MW, Carraro L, Moreira RO, Junior RF, Godoy‐Matos AF, et al. Evaluation of the sibutramine effect on satiety with a visual analogue scale in obese adolescents [Avaliação do efeito da sibutramina sobre a saciedade por escala visual analógica em adolescentes obesos]. Arquivos Brasileiros de Endocrinologia & Metabologia 2005;49(2):286‐90. - PubMed
-
- Godoy‐Matos A, Carraro L, Vieira A, Oliveira J, Guedes EP, Mattos L, et al. Treatment of obese adolescents with sibutramine: a randomized, double‐blind, controlled study. Journal of Clinical Endocrinology & Metabolism 2005;90(3):1460‐5. - PubMed
Kendall 2013 {published data only}
Maahs 2006 {published data only}
-
- Maahs D, Serna DG, Kolotkin RL, Ralston S, Sandate J, Qualls C, et al. Randomized, double‐blind, placebo‐controlled trial of orlistat for weight loss in adolescents. Endocrine Practice 2006;12(1):18‐28. - PubMed
Mauras 2012 {published data only}
-
- Benson M, Hossain J, Caulfield MP, Damaso L, Gidding S, Mauras N. Lipoprotein subfractions by ion mobility in lean and obese children. Journal of Pediatrics 2012;161:997‐1003. - PubMed
NCT00001723 {unpublished data only}
-
- Condarco TA, Sherafat‐Kazemzadeh R, McDuffie JR, Brady S, Salaita C, Sebring NG, et al. Long‐term follow‐up of a randomized, placebo‐controlled trial of orlistat in African‐American and Caucasian adolescents with obesity‐related comorbid conditions. Endocrine Reviews 2013.
Ozkan 2004 {published data only}
-
- Ozkan B, Bereket A, Turan S, Keskin S. Addition of orlistat to conventional treatment in adolescents with severe obesity. European Journal of Pediatrics 2004;163(12):738‐41. - PubMed
Prado 2012 {published data only (unpublished sought but not used)}
-
- Prado B, Gaete V, Corona F, Peralta E, Donoso P, Raimann X. Metabolic effects of metformin in obese adolescents at risk for type 2 diabetes mellitus [Efecto metabólico de la metformina en adolescentes obesas con riesgo de diabetes mellitus tipo 2]. Revista Chilena de Pediatría 2012;83(1):48‐57.
Rezvanian 2010 {published data only}
Srinivasan 2006 {published data only}
-
- Srinivasan S, Ambler GR, Baur LA, Garnett SP, Tepsa M, Yap F, et al. Randomized, controlled trial of metformin for obesity and insulin resistance in children and adolescents: improvement in body composition and fasting insulin. Journal of Clinical Endocrinology & Metabolism 2006;91(6):2074‐80. [DOI: 10.1210/jc.2006-0241] - DOI - PubMed
Van Mil 2007 {published data only}
-
- Mil EG, Westerterp KR, Kester AD, Delemarre‐van de Waal HA, Gerver WJ, Saris WH. The effect of sibutramine on energy expenditure and body composition in obese adolescents. Journal of Clinical Endocrinology & Metabolism 2007;92(4):1409‐14. - PubMed
Wiegand 2010 {published data only}
-
- Hübel H. Prospective randomised controlled study for the prevention of type 2 diabetes mellitus in obese children and adolescents, 2012 [Prospektive, randomisierte, kontrollierte Studie zur Prävention des Typ 2 Diabetes mellitus bei adipösen Kindern und Jugendlichen (Dissertation)]. www.diss.fu‐berlin.de/diss/servlets/MCRFileNodeServlet/FUDISS_derivate_000000010572/... (last accessed 20 May 2016).
-
- Wiegand S, l'Allemand D, Hübel H, Krude H, Bürmann M, Martus P, et al. Metformin and placebo therapy both improve weight management and fasting insulin in obese insulin‐resistant adolescents: a prospective, placebo‐controlled, randomized study. European Journal of Endocrinology 2010;163(4):585‐92. - PubMed
Wilson 2010 {published data only}
-
- Wilson DM, Abrams SH, Aye T, Lee PD, Lenders C, Lustig RH, et al. Metformin extended release treatment of adolescent obesity: a 48‐week randomized, double‐blind, placebo‐controlled trial with 48‐week follow‐up. Archives of Pediatrics & Adolescent Medicine 2010;164(2):116‐23. [DOI: 10.1001/archpediatrics.2009.264] - DOI - PMC - PubMed
Yanovski 2011 {published data only}
References to studies excluded from this review
Andelman 1967 {published data only}
-
- Andelman MB, Jones C, Nathan S. Treatment of obesity in underprivileged adolescents. Comparison of diethylpropion hydrochloride with placebo in a double‐blind study. Clinical Pediatrics 1967;6:327‐30. - PubMed
Ardizzi 1996 {published data only}
-
- Ardizzi A, Grugni G, Guzzaloni G, Moro D, Morabito F. Effects of protracted rhGH administration on body composition and intermediate metabolism in juvenile obesity. Acta Medica Auxologica 1996;28:103‐9.
Arman 2008 {published data only}
-
- Arman S, Sadramely MR, Nadi M, Koleini N. A randomized, double‐blind, placebo‐controlled trial of metformin treatment for weight gain associated with initiation of risperidone in children and adolescents. Saudi Medical Journal 2008;29:1130‐4. - PubMed
Bacon 1967 {published data only}
-
- Bacon GE, Lowrey GH. A clinical trial of fenfluramine in obese children. Current therapeutic research. Clinical and Experimental 1967;9:626‐30. - PubMed
Beyer 1980 {published data only}
-
- Beyer G, Huth K, Muller GM, Niemoller H, Raisp I, Vorberg G. The treatment of obesity with the appetite curbing agent mefenorex [Zur Behandlung Von Übergewicht mit dem Appetitzügler Mefenorex]. Medizinische Welt 1980;31:306‐9. - PubMed
Burgert 2008 {published data only}
-
- Burgert TS, Duran EJ, Goldberg‐Gell R, Dziura J, Yeckel CW, Katz S, et al. Short‐term metabolic and cardiovascular effects of metformin in markedly obese adolescents with normal glucose tolerance. Pediatric Diabetes 2008;9:567‐76. - PubMed
Canlorbe 1976 {published data only}
-
- Canlorbe P, Borniche P, Toublanc JE. Controlled trial of an anorectic (An 448) in the treatment of childhood obesity [Essai controle d'un anorexigene (An 448) dans le traitment de l'obesite de l'enfant]. La Nouvelle Presse Médicale 1976;5:1061‐2. - PubMed
Cannella 1968 {published data only}
-
- Cannella M, Scarpelli PT, Vailati G. The role of anorexic drugs in the treatment of obesity. Clinical data on the use of a new anorexic drug (Ro 6‐1343) [Il ruolo dei farmaci antioressici nel trattamento delle obesità. Rilievi clinici sull'impiego di un nuovo antioressico (Ro 6‐1343)]. Minerva Medica 1968;59:3472‐83. - PubMed
Casteels 2010 {published data only}
-
- Casteels K, Fieuws S, Helvoirt M, Verpoorten C, Goemans N, Coudyzer W, et al. Metformin therapy to reduce weight gain and visceral adiposity in children and adolescents with neurogenic or myogenic motor deficit. Pediatric Diabetes 2010;11:61‐9. - PubMed
Cayir 2015 {published data only}
-
- Cayir A, Turan MI, Gurbuz F, Kurt N, Yildirim A. The effect of lifestyle change and metformin therapy on serum arylesterase and paraoxonase activity in obese children. Journal of Pediatric Endocrinology and Metabolism 2015;28(5‐6):551‐6. - PubMed
CTRI/2011/10/002081 2011 {published data only}
-
- Biju KR, Patel KS. A clinical trial to study the effect of an Ayurvedic drug, Thriphalaguduchyadi Vati, in children with obesity. Clinical Trial Registry ‐ India 2011.
Danielsson 2007 {published data only}
-
- Danielsson P, Janson A, Norgren S, Marcus C. Impact sibutramine therapy in children with hypothalamic obesity or obesity with aggravating syndromes. Journal of Clinical Endocrinology and Metabolism 2007;92:4101‐6. - PubMed
Danilovich 2014 {published data only}
-
- Danilovich N, Mastrandrea LD, Cataldi L, Quattrin T. Methylphenidate decreases fat and carbohydrate intake in obese teenagers. Obesity (Silver Spring, Md.) 2014;22:781‐5. - PubMed
De Bock 2012 {published data only}
Delitala 1977 {published data only}
-
- Delitala G, Alagna S, Masala A. Mazindol (AN‐448): a new non‐amphetamine anorectic in the treatment of exogenous obesity [Il mazindolo (AN‐448): un nuovo anoressante non amfetaminico nel trattamento dell'obesita esogena]. Clinica Terapeutica 1977;81:547‐54. - PubMed
Diaz 2013 {published data only}
-
- Diaz M, Bassols J, Lopez‐Bermejo A, Zegher F, Ibanez L. Metformin treatment to reduce central adiposity after prenatal growth restraint: a placebo‐controlled pilot study in prepubertal children. Pediatric Diabetes. 2015;16(7):538‐45. - PubMed
-
- Diaz M, Ibanez L, Lopez‐Bermejo A, Sanchez‐Infantes D, Bassols J, Zegher F. Growth restraint before birth, weight catch‐up in infancy, and central adiposity in childhood: a placebo‐controlled pilot study of early metformin intervention. Hormone Research in Paediatrics 2013;80:116.
Di Natale 1973 {published data only}
-
- Natale B, Devetta M, Rossi L, Garlaschi C, Caccamo A, Guercia MJ, et al. Arginine infusion in obese children. Helvetica Paediatrica Acta 1973;28:331‐9. - PubMed
Doggrell 2006 {published data only}
-
- Doggrell SA. Sibutramine for obesity in adolescents. Expert Opinion on Pharmacotherapy 2006;7:2435‐8. - PubMed
EUCTR2009‐016921‐32‐ES {unpublished data only}
-
- Fundació Sant Joan de Déu. A phase II, randomized, double‐blind, placebo‐controlled, in parallel groups clinical trial to assess the safety and efficacy of dietary supplementation with tryptophan to achieve weight loss, and its neuropsychological effects in adolescents with obesity. clinicaltrials.gov/ct2/show/NCT02612259 Date first received: 17 November 2015. [NCT02612259]
EUCTR2012‐000038‐20‐DE {unpublished data only}
-
- Novo Nordisk A/S. A randomised, double‐blind, placebo‐controlled trial to assess safety, tolerability and pharmacokinetics of liraglutide in obese adolescent subjects aged 12 to 17 years. clinicaltrials.gov/ct2/show/NCT01789086 Date first received: 8 February 2013.
Fanghänel 2001 {published data only}
-
- Fanghänel G, Cortinas L, Sánchez‐Reyes L, Berber A. Second phase of a double‐blind study clinical trial on sibutramine for the treatment of patients suffering essential obesity: 6 months after treatment cross‐over. International Journal of Obesity and Related Metabolic Disorders: Journal of the International Association for the Study of Obesity 2001;25:741‐7. - PubMed
Faria 2002 {published data only}
-
- Faria AN, Ribeiro Filho FF, Lerário DDG, Kohlmann N, Ferreira SRG, Zanella MT. Effects of sibutramine on the treatment of obesity in patients with arterial hypertension [Efeitos da sibutramina no tratamento da obesidade em pacientes com hipertensão arterial]. Arquivos Brasileiros de Cardiologia 2002;78:172‐80. - PubMed
Ferguson 1986 {published data only}
-
- Ferguson JM. Fluoxetine‐induced weight loss in overweight, nondepressed subjects. American Journal of Psychiatry 1986;143:1496. - PubMed
Ferrara 2013 {published data only}
-
- Ferrara P, Bufalo F, Ianniello F, Franceschini A, Paolini Paoletti F, Massart F, et al. Diet and physical activity "defeated" Tuberil in treatment of childhood obesity. Minerva Endocrinologica 2013;38:181‐5. - PubMed
Fox 2015 {published data only}
Freemark 2007 {published data only}
-
- Freemark M. Pharmacotherapy of childhood obesity: an evidence‐based, conceptual approach. Diabetes Care 2007;30:395‐402. - PubMed
Galloway 1975 {published data only}
-
- Galloway DB, Logie AW, Petrie JC. Prolonged‐action fenfluramine in non‐diabetic patients with refractory obesity. Postgraduate Medical Journal 1975;51(Suppl 1):155‐7. - PubMed
Gamski 1968 {published data only}
-
- Gamski M, Komarnicka R. Regulation of appetite in obese subjects by means of neutrotropic drugs [Regulacja łaknienia u osób otyłych za pomoca leków neutrotropowych]. Polski Tygodnik Lekarski 1968;23:1688‐90. - PubMed
Garnett 2010 {published data only}
-
- Garnett SP, Baur LA, Noakes M, Steinbeck K, Woodhead HJ, Burrell S, et al. Researching effective strategies to improve insulin sensitivity in children and teenagers ‐ RESIST. A randomised control trial investigating the effects of two different diets on insulin sensitivity in young people with insulin resistance and/or pre‐diabetes. BMC Public Health 2010;10:575. - PMC - PubMed
-
- Garnett SP, Dunkley M, Ho M, Baur LA, Noakes M, Cowell CT. Optimum macronutrient content of the diet for adolescents with pre‐diabetes: RESIST, a randomised control trial (ACTRN12608000416392). Pediatric Diabetes 2012;13:23‐4.
-
- Tam C, Lu J, Gow M, Ho M, Cowell C, Baur L, et al. Changes in systemic inflammation in obese children with prediabetes after a 12 month lifestyle intervention; RESIST, a randomised control trial. Obesity Research and Clinical Practice 2013;7:e80.
Genova 1967 {published data only}
-
- Genova R, Massolo F, Venturelli, D. The lipolytic action of ACTH. 3. comparison of the lipolytic and corticoadrenal response to extrative and synthetic corticotropin in obese children [L'azione lipopolitica dell'ACTH. 3. Confronto tra la riposta lipopolitica e corticosurrenalica alla corticotropina estrattiva ed a quella sintetica nei bambini obesi]. Clinica Pediatrica 1967;49(6):277‐87. - PubMed
Gill 1977 {published data only}
-
- Gill E. Long‐term treatment of obesity with the anorectic agent tenuate as an adjuvant to reducing diet [Langzeitbehandlung der Adipositas mit dem anorektikum tenuate als adjuvans der reduktionsdiät]. Medizinische Welt 1977;28:2080‐1. - PubMed
Giovannini 1990 {published data only}
-
- Giovannini C, Ciucci E, Facchinetti F. Plasma levels of beta‐endorphin, ACTH and cortisol in obese patients subjected to several weight‐loss treatments [Livelli Plasmatici Di Beta‐Endorfinemia, Acth E Cortisolo In Pazienti Obesi Sottoposti A Differenti Trattamenti Dimagranti]. Recenti Progressi In Medicina 1990;81:301‐5. - PubMed
Godefroy 1968 {published data only}
-
- Godefroy G, Napolier A, Andriambao‐Damasy S. Study of the action of concentrated lucofen with prolonged effect [Etude de l'action du lucofène fort à effet prolongé]. Marseille Medical 1968;105:289‐93. - PubMed
Goldrick 1973 {published data only}
-
- Goldrick RB, Havenstein N, Whyte HM. Effects of caloric restriction and fenfluramine on weight loss and personality profiles of patients with long standing obesity. Australian And New Zealand Journal of Medicine 1973;3:131‐41. - PubMed
Goldstein 1993 {published data only}
-
- Goldstein DJ, Rampey AH, Dornseif BE, Levine LR, Potvin JH, Fludzinski LA. Fluoxetine: a randomized clinical trial in the maintenance of weight loss. Obesity Research 1993;1:92‐8. - PubMed
González Barranco 1974 {published data only}
-
- González Barranco J, Rull JA, Lozano Castañeda O. L‐Thyroxine propylhexedrine in the treatment of obesity. Crossed double blind study [Propilhexedrina L‐tiroxina en el tratamiento de la obesidad. Estudio doble ciego gruzado]. La Prensa Médica Mexicana 1974;39:298‐9. - PubMed
Griboff 1975 {published data only}
-
- Griboff SI, Berman R. A double blind clinical evaluation of a phenylpropanolamine‐caffeine‐vitamine combination and a placebo in the treatment of exogenous obesity. Current Therapeutic Research, Clinical and Experimental 1975;17:535‐43. - PubMed
Grube 2014 {published data only}
-
- Grube B, Chong WF, Chong PW, Riede L. Weight reduction and maintenance with Iqp‐Pv‐101: a 12‐week randomized controlled study with a 24‐week open label period. Obesity 2014;22:645‐51. - PubMed
Guazzelli 1987 {published data only}
-
- Guazzelli R, Piazzini M, Conti C, Papi L, Strazzulla G, Matassi L. Clinical use of mazindol in the treatment of essential obesity [Impiego clinico del mazindolo nel trattamento dell'obesità essenziale]. Clinica Terapeutica 1987;120:385‐91. - PubMed
Gwinup 1967 {published data only}
-
- Gwinup G, Poucher R. A controlled study of thyroid analogs in the therapy of obesity. American Journal of the Medical Sciences 1967;254:416‐20. - PubMed
Halpern 2006 {published data only}
-
- Halpern A, Mancini MC, Cercato C, Villares SMF, Costa AP. Effects of growth hormone on anthropometric and metabolic parameters in android obesity [Efeito Do Hormônio De Crescimento Sobre Parâmetros Antropométricos E Metabólicos Na Obesidade Andróide]. Arquivos Brasileiros de Endocrinologia & Metabologia 2006;50:68‐73. - PubMed
Hamilton 2003 {published data only}
-
- Hamilton J, Cummings E, Zdravkovic V, Finegood D, Daneman D. Metformin as an adjunct therapy in adolescents with type 1 diabetes and insulin resistance: a randomized controlled trial. Diabetes Care 2003;26:138‐43. - PubMed
Hansen 2001 {published data only}
-
- Hansen D, Astrup A, Toubro S, Finer N, Kopelman P, Hilsted J, et al. Predictors of weight loss and maintenance during 2 years of treatment by sibutramine in obesity. Results from the European multi‐centre Storm trial (Sibutramine Trial of Obesity Reduction and Maintenance). International Journal of Obesity and Related Metabolic Disorders : Journal of the International Association for the Study of Obesity 2001;25:496‐501. - PubMed
Haug 1973 {published data only}
-
- Haug E, Myklebust R. The therapeutic effect of fenfluramine on body weight in overweight patients. A controlled clinical study [Den terapeutiske effekt av fenfluramin på kroppsvekten hos overvektige. En kontrollert klinisk undersøkelse]. Tidsskrift For Den Norske Lægeforening : Tidsskrift For Praktisk Medicin, Ny Række 1973;93(35):2545‐9. - PubMed
Hawkins 2012 {published data only}
-
- Hawkins FP. Metformin and placebo therapy in adjunct with lifestyle intervention both improve weight loss and insulin resistance in obese adolescents. Archives of Disease in Childhood ‐ Education and Practice 2012;97:79‐80. - PubMed
Honzak 1976 {published data only}
-
- Honzak R, Rath R, Vondra K. Effect of cafilon in the treatment of obesity (author's translation). Ceskoslovenska Gastroenterologie A Vyziva 1976;30:155‐61. - PubMed
Hooper 1972 {published data only}
-
- Hooper AC. Comparison of fenfluramine (with ad libitum food intake) with 1,000 calorie diet in obesity. Journal of the Irish Medical Association 1972;65:35‐7. - PubMed
Huston 1966 {published data only}
-
- Huston JR. Obesity: phenmetrazine effect without dietary restriction. Ohio State Medical Journal 1966;62:805‐7. - PubMed
IRCT2013021012421N1 {published data only}
IRCT2014020116435N1 {unpublished data only}
-
- Shahrekord University of Medical Sciences. The effect of folic acid on homocysteine and plasma insulin levels in weight gain and obesity in children and adolescents. en.search.irct.ir/view/16869 Date first received: 23 May 2014.
Israsena 1980 {published data only}
-
- Israsena T, Israngkura M, Srivuthana S. Treatment of childhood obesity. Journal of the Medical Association of Thailand 1980;63:433‐7. - PubMed
James 2000 {published data only}
-
- James WP, Astrup A, Finer N, Hilsted J, Kopelman P, Rössner S, et al. Effect of sibutramine on weight maintenance after weight loss: a randomised trial. Storm study group. Sibutramine trial of obesity reduction and maintenance. Lancet 2000;356:2119‐25. - PubMed
Kasa‐Vubu 2008 {published data only}
-
- Kasa‐Vubu JZ. Metformin as a weight‐loss tool in "at‐risk" obese adolescents: a magic bullet?. Journal of Pediatrics 2008;152(6):750‐2. - PubMed
Kay 2001 {published data only}
-
- Kay JP, Alemzadeh R, Langley G, D'angelo L, Smith P, Holshouser S. Beneficial effects of metformin in normoglycemic morbidly obese adolescents. Metabolism: Clinical and Experimental 2001;50:1457‐61. - PubMed
Kelly 2012 {published data only}
Kelly 2013a {published data only}
-
- Kelly AS. The role of glucagon‐like peptide‐1 receptor agonists for the treatment of adolescent obesity. Expert Review of Endocrinology and Metabolism 2013;8:315‐7. - PubMed
Kelly 2013b {published data only}
Kendall 2014 {published data only}
-
- Kendall DL, Amin R, Clayton PE. Metformin in the treatment of obese children and adolescents at risk of type 2 diabetes. Paediatric Drugs 2014;16:13‐20. - PubMed
Klein 2006 {published data only}
-
- Klein DJ, Cottingham EM, Sorter M, Barton BA, Morrison JA. A randomized, double‐blind, placebo‐controlled trial of metformin treatment of weight gain associated with initiation of atypical antipsychotic therapy in children and adolescents. American Journal of Psychiatry 2006;163:2072‐9. - PubMed
Kneebone 1968 {published data only}
-
- Kneebone GM. Fenfluramine in the treatment of obesity. Medical Journal of Australia 1968;2:833‐5. - PubMed
Knoll 1975 {published data only}
-
- Knoll G, Spahn U, Plenert W. Therapy of obesity in childhood. Kinderarztliche Praxis 1975;43:412‐26. - PubMed
Komarnicka 1975 {published data only}
-
- Komarnicka R, Gorzko M. Results of treatment of obesity with low calorie diet and additional drugs (author's translation) [Wyniki leczenia otyłości dieta niskokaloryczna i lekami wspomagajacymi]. Przeglad Lekarski 1975;32:814‐7. - PubMed
Komorowski 1982 {published data only}
-
- Komorowski JM, Zwaigzne‐Raczynska J, Owczarczyk I, Golebiowska M, Zarzycki J. Effect of mazindol (Teronac) on various hormonal indicators in children with simple obesity. Pediatria Polska 1982;57:241‐6. - PubMed
Kreze 1967 {published data only}
-
- Kreze A, Kiselá J. Biguanides and the treatment of obesity [Biguanidy a terapia obezity]. Bratislavské Lekárske Listy 1967;48:184‐9. - PubMed
Lamberto 1993 {published data only}
-
- Lamberto M, Novi RF, Mantovan M. Fluoxetine and obesity [Fluoxetina ed obesità]. Minerva Endocrinologica 1993;18:41‐3.. - PubMed
Leite 1971 {published data only}
-
- Leite AC, Liepen LL, Costa VP. Clinical trial of stimsem thozalinone in the treatment of obese patients [Ensaio clĩnico com o stimsem thozalinone no tratamento de pacientes obesos]. Revista Brasileira De Medicina 1971;28:475‐8. - PubMed
Lewis 1978 {published data only}
-
- Lewis EA, Adeleye GI, Ukponmwan OO. Comparison of fenfluramine and placebo on obese Nigerians. Nigerian Medical Journal 1978;8:104‐7. - PubMed
Libman 2015 {published data only}
-
- Libman IM, Miller KM, Dimeglio LA, Bethin KE, Katz ML, Shah A, et al. Effect of metformin added to insulin on glycemic control among overweight/obese adolescents with type 1 diabetes a randomized clinical trial. JAMA 2015;314:2241‐50. - PubMed
Liebermeister 1969 {published data only}
-
- Liebermeister H, Probst G, Jahnke K. Experience with the appetite depressant, fenfluramine hydrochloride, in adiposity [Erfahrungen mit dem Appetithemmer Fenfluraminhydrochlorid bei der Adipositas]. Medizinische Klinik 1969;64:1201‐7. - PubMed
Liu 2013 {published data only}
-
- Liu AG, Smith SR, Fujioka K, Greenway FL. The effect of leptin, caffeine/ephedrine, and their combination upon visceral fat mass and weight loss. Obesity 2013;21:1991‐6. - PubMed
Lorber 1966 {published data only}
Love‐Osborne 2008 {published data only}
Maclay 1977 {published data only}
-
- Maclay WP, Wallace MG. A multi‐centre general practice trial of mazindol in the treatment of obesity. Practitioner 1977;218:431‐4. - PubMed
Malchow‐Møller 1980 {published data only}
-
- Malchow‐Møller A, Larsen S, Hey H, Stokholm KH, Juhl E, Quaade F. Effect of elsinore tablets in the treatment of obesity. A controlled clinical trial. Ugeskrift For Laeger 1980;142:1496‐9. - PubMed
Marques 2016 {published data only}
-
- Marques P, Limbert C, Oliveira L, Santos MI, Lopes L. Metformin effectiveness and safety in the management of overweight/obese nondiabetic children and adolescents: metabolic benefits of the continuous exposure to metformin at 12 and 24 months. International Journal of Adolescent Medicine and Health 2016 Feb 19;[Epub ahead of print]. - PubMed
McDuffie 2002 {published data only}
-
- McDuffie JR, Calis KA, Uwaifo GI, Sebring NG, Fallon EM, Hubbard VS, et al. Three‐month tolerability of orlistat in adolescents with obesity‐related comorbid conditions. Obesity Research 2002;10:642‐50. - PubMed
Molnár 2000 {published data only}
-
- Molnár D, Török K, Erhardt E, Jeges S. Safety and efficacy of treatment with an ephedrine/caffeine mixture. The first double‐blind placebo‐controlled pilot study in adolescents. International Journal of Obesity and Related Metabolic Disorders 2000;24:1573‐8. - PubMed
Muls 2001 {published data only}
-
- Muls E, Kolanowski J, Scheen A, Gaal L, Obelhyx Study Group. The effects of orlistat on weight and on serum lipids in obese patients with hypercholesterolemia: a randomized, double‐blind, placebo‐controlled, multicentre study. International Journal of Obesity and Related Metabolic Disorders 2001;25:1713‐21. - PubMed
Nadeau 2015 {published data only}
Nathan 2016 {published data only}
NCT00076362 {unpublished data only}
-
- Novartis Pharmaceuticals. Pediatric hypothalamic obesity. clinicaltrials.gov/ct2/show/NCT00076362 Date first received: 21 January 2004.
NCT00284557 {unpublished data only}
-
- Emory University. A primary care behavioral approach for addressing childhood overweight. clinicaltrials.gov/ct2/show/NCT00284557 Date first received: 30 January 2006.
NCT00775164 {unpublished data only}
-
- University of Minnesota ‐ Clinical and Translational Science Institute. Pioglitazone therapy in obese children with insulin resistance: a randomized, controlled pilot study. clinicaltrials.gov/ct2/show/NCT00775164 Date first received: 16 October 2008.
NCT00845559 {unpublished data only}
-
- Yale University. The effects of exenatide on post‐meal sugar peaks and vascular health in obese/pre‐diabetic young adults. clinicaltrials.gov/ct2/show/NCT00845559 Date first received: 16 February 2009.
NCT01023139 {unpublished data only}
-
- Brooke Army Medical Center. "Efficacy in adolescents of continued behavior modification following a six month sibutramine‐based weight management intervention" (AOS). clinicaltrials.gov/ct2/show/NCT01023139 Date first received: 1 December 2009.
NCT01061775 {unpublished data only}
-
- Children's Hospitals and Clinics of Minnesota. Effects of exenatide on hypothalamic obesity. clinicaltrials.gov/ct2/show/NCT01061775 Date first received: 2 February 2010.
NCT01107808 {published data only}
-
- University of Alabama at Birmingham. Calcium, vitamin D and metformin to treat insulin resistance in obese African American adolescent females. clinicaltrials.gov/ct2/show/NCT01107808 Date first received: 1 April 2010.
NCT01169103 {published data only}
-
- NCT01169103. Effect of recombinant human growth hormone (rhGH) on abdominal fat and cardiovascular risk in obese girls. clinicaltrials.gov/ct2/show/NCT01169103 Date first received: 22 July 2010.
NCT01242241 {unpublished data only}
-
- Baylor College of Medicine. Propofol in obese children. clinicaltrials.gov/ct2/show/NCT01242241 Date first received: 15 November 2010.
NCT01329367 {unpublished data only}
-
- Clinica Universidad de Navarra, Universidad de Navarra. Nutrigenomics and children obesity: a moderate weight loss intervention study (NUGENOI). clinicaltrials.gov/ct2/show/NCT01329367 Date first received: 20 January 2011.
NCT01332448 {unpublished data only}
-
- GlaxoSmithKline. Meta‐analysis of orlistat laboratory data from placebo‐controlled clinical trials. clinicaltrials.gov/ct2/show/NCT01332448 Date first received: 7 April 2011.
NCT01410604 {unpublished data only}
-
- Hospital Regional de Alta Especialidad del Bajio. Adipokines in obese adolescents with insulin resistance. clinicaltrials.gov/ct2/show/NCT01410604 Date first received: 4 August 2011.
NCT01456221 {unpublished data only}
-
- Coordinación de Investigación en Salud, Mexico. Intervention study with omega‐3 fatty acids for weight loss and insulin resistance in adolescents (O3WLIRADOL). clinicaltrials.gov/ct2/show/NCT01456221 Date first received: 12 October 2011.
NCT01910246 {unpublished data only}
-
- University of California, San Francisco. Cardiovascular effects of metformin on obesity. clinicaltrials.gov/ct2/show/NCT01910246 Date first received: 22 April 2013.
NCT02022956 {unpublished data only}
-
- Arena Pharmaceuticals. Single dose study to determine the safety, tolerability, and pharmacokinetic properties of lorcaserin hydrochloride (BELVIQ) in obese adolescents from 12 to 17 years of age. clinicaltrials.gov/ct2/show/NCT02022956 Date first received: 23 December 2013.
NCT02063802 {published data only}
-
- Laboratorios Silanes S.A. de C.V. Metformin vs conjugated linoleic acid and an intervention program with healthy habits in obese children. clinicaltrials.gov/ct2/show/NCT02063802 Date first received: 12 February 2014.
NCT02186652 {unpublished data only}
-
- Duke University. PK study with pantoprazole in obese children and adolescents (PAN01). clinicaltrials.gov/ct2/show/NCT02186652 Date first received: 3 July 2014.
NCT02378259 {published data only}
-
- NCT02378259. Randomized clinical trial; medical vs bariatric surgery for adolescents (13‐16 y) with severe obesity. clinicaltrials.gov/ct2/show/NCT02378259 Date first received: 26 August 2014.
NCT02398669 {published data only}
-
- Eisai Inc. A single dose pharmacokinetic study of lorcaserin hydrochloride in obese pediatric subjects 6 to 11 years of age. clinicaltrials.gov/ct2/show/NCT02398669 Date first received: 20 March 2015.
NCT02438020 {unpublished data only}
-
- Juan Pablo, C.‐C, Hospital Central "Dr. Ignacio Morones, P, Universidad Autonoma De San Luis. P. Study of efficacy of metformin in the treatment of acanthosis nigricans in children with obesity. clinicaltrials.gov/ct2/show/NCT02438020 Date first received: 3 May 2015.
NCT02515773 {unpublished data only}
-
- NCT02515773. Metformin for overweight & obese children and adolescents with BDS treated with SGAs. clinicaltrials.gov/ct2/show/NCT02515773 Date first received: 31 July 2015.
Nwosu 2015 {published data only}
O'connor 1995 {published data only}
-
- O'connor HT, Richman RM, Steinbeck KS, Caterson ID. Dexfenfluramine treatment of obesity: a double blind trial with post trial follow up. International Journal of Obesity and Related Metabolic Disorders : Journal of the International Association for the Study of Obesity 1995;19:181‐9. - PubMed
Park 2010 {published data only}
-
- Park MH, Kinra S. Metformin treatment for adolescent obesity has limited long‐term benefits. Journal of Pediatrics 2010;157:172. - PubMed
Pedrinola 1994 {published data only}
-
- Pedrinola F, Cavaliere H, Lima N, Medeiros‐Neto G. Is Dl‐Fenfluramine a potentially helpful drug therapy in overweight adolescent subjects?. Obesity Research 1994;2:1‐4. - PubMed
Persson 1973 {published data only}
-
- Persson I, Andersen U, Deckert T. Treatment of obesity with fenfluramine. European Journal of Clinical Pharmacology 1973;6:93‐7. - PubMed
Plauchu 1967a {published data only}
-
- Plauchu M, Montgolfier R, Noel P. Clinical study on a new appetite depressant Mg 559 [Etude clinique d'un anorexigène récent: le MG 559]. Lyon Medical 1967;217(15):1105‐16. - PubMed
Plauchu 1967b {published data only}
-
- Plauchu M, Kofman J, Chapuy H. Clinical study of a new anorexigen: RD 354 (Hepatrol) [Etude clinique d'un nouvel anorexigène: le RD 354]. Lyon Medical 1967;217(17):1247‐61. - PubMed
Plauchu 1972 {published data only}
-
- Plauchu M, Paffoy JC, Philippe LP, Nove‐Josserand G, Chollet A, Valancogne C. Clinical study of a current anorexigenic agent: T.D [Etude clinique d'un anorexigène récent: le T.D]. Lyon Medical 1972;228(15):291‐4. - PubMed
Pugnoli 1978 {published data only}
-
- Pugnoli C, Bandini L, Caronna S, Ciarlini E, Magnati G, Strata A, et al. Clinical trial of a new long‐acting fenfluramine in the treatment of obesity [Ricerca Clinica Controllata Su Un Nuovo Preparato Di Fenfluramina 'A Lenta Cessione' Nel Trattamento Dell'obesita]. Giornale Di Clinica Medica 1978;59:486‐510. - PubMed
Rauh 1968 {published data only}
-
- Rauh JL, Lipp R. Chlorphentermine as an anorexigenic agent in adolescent obesity. Report of its efficacy in a double‐blind study of 30 teen‐agers. Clinical Pediatrics 1968;7:138‐40. - PubMed
Resnick 1967 {published data only}
Rodos 1969 {published data only}
-
- Rodos JJ. The treatment of obesity with voranil (Su‐10568), a new non‐amphetamine anorexigenic agent. Journal of the American Osteopathic Association 1969;69:161‐4. - PubMed
Rodriguez 2007 {published data only}
-
- Rodriguez JE, Shearer B, Slawson DC. Metformin therapy and diabetes prevention in adolescents who are obese. American Family Physician 2007;76:1357‐9. - PubMed
Roed 1980 {published data only}
-
- Roed P, Hansen PW, Bidstrup B, Kaern M, Helles A, Petersen KP. Elsinore banting tablets. A controlled clinical trial in general practice [Helsingor‐slankepiller: En kontrolleret klinisk undersogelse i almen praksis]. Ugeskrift For Laeger 1980;142:1491‐5. - PubMed
Roginsky 1966 {published data only}
-
- Roginsky MS, Barnett J. Double‐blind study of phenethyldiguanide in weight control of obese nondiabetic subjects. American Journal of Clinical Nutrition 1966;19:223‐6. - PubMed
Sabuncu 2004 {published data only}
-
- Sabuncu T, Ucar E, Birden F, Yasar O. The effect of 1‐yr sibutramine treatment on glucose tolerance, insulin sensitivity and serum lipid profiles in obese subjects. Diabetes, Nutrition & Metabolism ‐ Clinical & Experimental 2004;17:103‐7. - PubMed
Sainani 1973 {published data only}
-
- Sainani GS, Fulambarkar AM, Khurana BK. A double‐blind clinical trial of fenfluramine in the treatment of obesity. British Journal of Clinical Practice 1973;27:136‐8. - PubMed
Scavo 1976 {published data only}
-
- Scavo D, Giovannini C, Sellini M, Fierro A. Therapy of obesity in childhood and adolescence. Comparison of results obtained with dietary and drug treatment [La terapia dell'obesita nell'infanzia e nell'adolescenza]. Clinica Terapeutica 1976;79:205‐18. - PubMed
Shutter 1966 {published data only}
-
- Shutter L, Garell DC. Obesity in children and adolescents: a double‐blind study with cross‐over. Journal of School Health 1966;36:273‐5. - PubMed
Spence 1966 {published data only}
-
- Spence AW, Medvei VC. Fenfluramine in the treatment of obesity. British Journal of Clinical Practice 1966;20:643‐4. - PubMed
Spranger 1963 {published data only}
-
- Spranger J. Anorexigenic drugs in the treatment of obesity in children. An enlarged double‐blind study with chlorphentermin. Munchener Medizinische Wochenschrift (1950) 1963;105:1338‐41. - PubMed
Spranger 1965 {published data only}
-
- Spranger J. Phentermine resinate in obesity. Clinical trial of Mirapront in adipose children [Phentermin‐Resinat bei Fettsucht. Klinische Prüfung von Mirapront bei adipösen Kindern]. Münchener medizinische Wochenschrift (1950) 1965;107:1833‐4. - PubMed
Sproule 1969 {published data only}
-
- Sproule BC. Double‐blind trial of anorectic agents. Medical Journal of Australia 1969;1:394‐5. - PubMed
Stewart 1970 {published data only}
-
- Stewart DA, Bailey JD, Patell H. Tenuate dospan as an appetite suppressant in the treatment of obese children. Applied Therapeutics 1970;12:34‐6. - PubMed
Sukkari 2010 {published data only}
-
- Sukkari SR, Sasich LD, Humaidan AS, Burikan O. Analysis of metformin treatment for adolescent obesity at 48 rather than 24 weeks after treatment cessation. Archives of Pediatrics & Adolescent Medicine 2010;164:678‐9 (author reply). - PubMed
TODAY study group 2013 {published data only}
-
- Arslanian SA, Pyle L, White NH, Bacha F, Caprio S, Haymond MW, et al. Racial disparity in maintenance of glycemic control in the TODAY trial: does insulin sensitivity, beta‐cell function, or both play a role?. Diabetes 2013;62:A338.
Tong 2005 {published data only}
-
- Tong NW, Ran XW, Li QF, Tang BD, Li R, Yang FY, et al. Effects of sibutramine on blood glucose and lipids, body fat mass and insulin resistance in obese patients: a multi‐center clinical trial. Zhonghua Nei Ke Za Zhi 2005;44:659‐63. - PubMed
Toubro 2001 {published data only}
-
- Toubro S, Hansen DL, Hilsted JC, Porsborg PA, Astrup AV, James WPT, et al. The effect of sibutramine for the maintenance of weight loss: a randomised, clinical, controlled study [Effekt af sibutramin til vægttabsvedligeholdelse: en randomiseret klinisk kontrolleret undersøgelse]. Ugeskrift for Laeger 2001;163(21):2935‐40. - PubMed
Tsai 2006 {published data only}
-
- Tsai AG, Wadden TA, Berkowitz RI. Pharmacotherapy for overweight adolescents. Obesity Management 2006;2:98‐102.
Van Seters 1982 {published data only}
-
- Seters AP, Bouwhuis‐Hoogerwerf ML, Goslings BM, Nieuwkoop L, Slooten H, Struijk‐Wielinga T. Long‐term treatment of patients with obesity using mazindol and a reducing diet. Nederlands Tijdschrift Voor Geneeskunde 1982;126:990‐4. - PubMed
Warren‐Ulanch 2008 {published data only}
-
- Warren‐Ulanch J. Metformin may aid weight loss in overweight teenage girls. Journal of Pediatrics 2008;153:725‐6. - PubMed
Weintraub 1984 {published data only}
-
- Weintraub M, Hasday JD, Mushlin AI, Lockwood DH. Double‐blind clinical trial in weight control. Use of fenfluramine and phentermine alone and in combination. Archives of Internal Medicine 1984;144:1143‐8. - PubMed
Yanovski 2003 {published data only}
-
- Yanovski JA, Yanovski SZ. Treatment of pediatric and adolescent obesity. JAMA 2003;289:1851‐3. - PubMed
Yu 2013 {published data only}
-
- Yu CC, Li AM, Chan KO, Chook P, Kam JT, Au CT, et al. Orlistat improves endothelial function in obese adolescents: a randomised trial. Journal of Paediatrics and Child Health 2013;49:969‐72. - PubMed
References to studies awaiting assessment
Golebiowska 1981 {published data only}
-
- Golebiowska M, Chlebna‐Sokol D, Kobierska I, Konopinska A, Malek M, Mastalska A, et al. Clinical evaluation of teronac (mazindol) in the treatment of obesity in children. Part II. Anorectic properties and side effects (author's translation). Przeglad Lekarski 1981;38:355‐8. - PubMed
Linquette 1971 {published data only}
-
- Linquette A, Fossati, P. Hunger control with benzphetamine hydrochloride in the treatment of obesity [Le contrôle de la faim par le chlorydrate de benzphétamine dans le traitement de l'obésité]. Lille Medical 1971;16(Suppl 2):620‐4. - PubMed
NCT00934570 {unpublished data only}
-
- Clarson CL, Brown H, Dejesus S, Jackman M, Mahmud FH, Prapavessis H, et al. Structured lifestyle intervention with metformin extended release or placebo in obese adolescents. Diabetes. 2013; Vol. 62:A337‐8.
NCT01487993 {unpublished data only}
Smetanina 2015 {published data only}
-
- Smetanina N, Seibokaite A, Valickas R, Kuprionis G, Grigoniene JJ, Rokaite R, et. al. Metformin in combination with lifestyle changes effectively reduces BMI and waist circumference in overweight/obese children and adolescents. Hormone Research in Paediatrics 2015;84:229.
References to ongoing studies
EUCTR2010‐023061‐21 {unpublished data only}
-
- Pastor MB, Canete MD, Hoyos R, Latorre M, Vazquez‐Cobela R, Rangel‐Huerta OD, et al. Metformin in the treatment of obese children shows differential response according to pubertal stage. Acta Physiologica. 2014; Vol. 212:36‐7.
EUCTR2015‐001628‐45‐SE {unpublished data only}
-
- A study with lifestyle intervention and study medication once weekly or lifestyle intervention and placebo in adolescents with obesity to explore differences between groups with regard to change in BMI. Ongoing study Trial start date: not givenTrial completion date: not given.
NCT00889876 {unpublished data only}
-
- Effect of exercise or metformin on nocturnal blood pressure and other risk factors for CVD among obese adolescents. Ongoing study Trial start date: February 2009Trial completion date: December 2012 (estimated).
NCT01677923 {unpublished data only}
-
- Obesity in children and adolescents: associated risks and early intervention (OCA). Ongoing study Trial start date: May 2013Trial completion date: December 2015.
NCT01859013 {unpublished data only}
-
- Topiramate in Adolescents with Severe Obesity. Ongoing study Trial start date: June 2013Trial completion date: December 2015.
NCT02273804 {unpublished data only}
-
- Topiramate and Severe Obesity (TOBI). Ongoing study Trial start date: June 2015Trial completion date: December 2020.
NCT02274948 {unpublished data only}
-
- Use of Metformin in Treatment of Childhood Obesity. Ongoing study Trial start date: July 2014Trial completion date: February 2016.
NCT02496611 {unpublished data only}
-
- Enhancing Weight Loss Maintenance With GLP‐1RA (BYDUREON™) in Adolescents with Severe Obesity. Ongoing study Trial start date: December 2015Trial completion date: July 2020.
Additional references
Aggarwal 2014
-
- Aggarwal A, Puri K, Thangada S, Zein N, Alkhouri N. Nonalcoholic fatty liver disease in children: recent practice guidelines, where do they take us?. Current Pediatric Reviews 2014;10(2):151‐61. - PubMed
Alonso 1995
-
- Alonso J, Prieto L, Anto JM. The Spanish version of the SF 36 Health Survey (the SF 36 health questionnaire): an instrument for measuring clinical results. Medicina Clinica 1995;104:771‐6. - PubMed
Barlow 1998
-
- Barlow SE, Dietz WH. Maternal and Child Health Resources and Services Administration and Department of Health and Human Services. Obesity evaluation and treatment: expert committee recommendations. Pediatrics 1998;102:E29. - PubMed
Bayer 2011
-
- Bayer O, Krüger H, Kries R, Toschke AM. Factors associated with tracking of BMI: a meta‐regression analysis on BMI tracking. Obesity 2011;19(5):1069‐76. - PubMed
Berardis 2014
Bocca 2013
-
- Bocca G, Ongering EC, Stolk RP, Sauer PJ. Insulin resistance and cardiovascular risk factors in 3‐ to 5‐year‐old overweight or obese children. Hormone Research in Paediatrics 2013;80(3):201‐6. - PubMed
Boland 2015
Borkan 1982
-
- Borkan GA, Gerzof SG, Robbins AH, Hults DE, Silbert CK, Silbert JE. Assessment of abdominal fat content by computed tomography. American Journal of Clinical Nutrition 1982;36(1):172‐7. - PubMed
Bouza 2012
-
- Bouza C, Lopez‐Cuadrado T, Gutierrez‐Torres LF, Amate J. Efficacy and safety of metformin for treatment of overweight and obesity in adolescents: an updated systematic review and meta‐analysis. Obesity Facts 2012;5:753‐65. - PubMed
Calloway 1988
-
- Calloway CW, Chumlea WC, Bouchard C. Circumferences. In: Lohman TC, Roche AR, Martorell R editor(s). Anthropometric Standardization Manual. Campaign, III. Champaign, IL: Human Kinetics Publishers, 1988:39‐64.
Catoira 2010
-
- Catoira N, Nagel M, Girolamo G, Gonzalez CD. Pharmacological treatment of obesity in children and adolescents: current status and perspectives. Expert Opinion on Pharmacotherapy 2010;11(18):2973‐83. - PubMed
Cheung 2013
CMO 2014
-
- Chief Medical Officer. Chief Medical Officer annual report: surveillance volume 2012. www.gov.uk/government/publications/chief‐medicalofficer‐annual‐report‐su... 27 March 2014.
Cole 1995
Cole 2000
Cole 2005
-
- Cole TJ, Faith MS, Pietrobelli A, Heo M. What is the best measure of adiposity change in growing children: BMI, BMI %, BMI z‐score or BMI centile?. European Journal of Clinical Nutrition 2005;59:419‐25. - PubMed
Cole 2012
-
- Cole TJ, Lobstein T. Extended international (IOTF) body mass index cut offs for thinness, overweight and obesity. Pediatric Obesity 2012;7:284‐94. - PubMed
Colquitt 2016
-
- Colquitt JL, Loveman E, O'Malley C, Azevedo LB, Mead E, Al‐Khudairy L, et al. Diet, physical activity, and behavioural interventions for the treatment of overweight or obesity in preschool children up to the age of 6 years. Cochrane Database of Systematic Reviews 2016, Issue 3. [DOI: 10.1002/14651858.CD012105] - DOI - PMC - PubMed
Czernichow 2010
-
- Czernichow S, Lee CM, Barzi F, Green F. Efficacy of weight loss drugs on obesity and cardiovascular risk factors in obese adolescents: a meta‐analysis of randomized controlled trials. Obesity Reviews 2010;11(2):150‐8. - PubMed
De Onis 2010
-
- Onis M, Blossner M. Borghi, E. Global prevalence and trends of overweight and obesity among preschool children. American Journal of Clinical Nutrition 2010;92:1257‐64. - PubMed
Derogatis 1983
-
- Derogatis LR, Melisaratos N. The Brief Symptom Inventory: an introductory report. Psychological Medicine 1983;13:595‐605. - PubMed
Dietz 1998
-
- Dietz, WH. Health consequences of obesity in youth: childhood predictors of adult disease. Paediatrics 1998;101(3S):518‐25. - PubMed
Ebbeling 2002
-
- Ebbeling CB, Pawlak DB, Ludwig DS. Childhood obesity: public‐health crisis, common sense cure. Lancet 2002;360(9331):473‐82. - PubMed
Ells 2015a
Ells 2015b
Falaschetti 2010
Fernandez 2004
-
- Fernandez JR, Redden DT, Pietrobelli A, Allison DB. Waist circumference percentiles in nationally representative samples of African‐American, European‐American, and Mexican‐American children and adolescent. Journal of Pediatrics 2004;145:439‐44. - PubMed
Fishbein 2001
-
- Fishbein MH, Stevens WR. Rapid MRI using a modified Dixon technique: a non‐invasive and effective method for detection and monitoring of fatty metamorphosis of the liver. Pediatric Radiology 2001;31(11):806‐9. - PubMed
Fishbein 2003
-
- Fishbein MH, Miner M, Mogren C, Chalekson J. The spectrum of fatty liver in obese children and the relationship of serum aminotransferases to severity of steatosis. Journal of Pediatric Gastroenterology and Nutrition 2003;36(1):54‐61. - PubMed
Freedman 1999
-
- Freedman DS, Dietz WH, Srinivasan SR, Berenson GS. The relation of overweight to cardiovascular risk factors among children and adolescents: the Bogalusa Heart Study. Pediatrics 1999;103(6 Pt 1):1175‐82. - PubMed
Freedman 2006
-
- Freedman DS, Khan LK, Serdula MK, Ogden CL, Dietz WH. Racial and ethnic differences in secular trends for childhood BMI, weight, and height. Obesity 2006;14:301‐8. - PubMed
Fuller 1992
-
- Fuller NJ, Jebb SA, Laskey MA, Coward WA, Elia M. Four‐component model for the assessment of body composition in humans: comparison with alternative methods, and evaluation of the density and hydration of fat‐free mass. Clinical Science (London) 1992;82:687‐93. - PubMed
Glenny 1997
-
- Glenny A M. O'Meara S, Melville A, sheldon TA, Wilson C. The treatment and prevention of obesity: a systematic review of the literature. International Journal of Obesity & Related Metabolic Disorders: Journal of the International Association for the Study of Obesity 1997;21(9):715‐37. - PubMed
Halford 2010
-
- Halford JC, Boyland EJ, Blundell JE, Kirkham TC, Harrold JA. Pharmacological management of appetite expression in obesity. Nature Reviews. Endocrinology 2010;6(5):255‐69. - PubMed
Halpern 2010
Hansen 1998
-
- Hansen DL, Toubro S, Stock MJ, Macdonald IA, Astrup A. Thermogenic effects of sibutramine in humans. American Journal of Clinical Nutrition 1998;68:1180‐6. - PubMed
Higgins 2002
-
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. - PubMed
Higgins 2003
Higgins 2009
Higgins 2011a
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
Hróbjartsson 2013
-
- Hróbjartsson A, Thomsen AS, Emanuelsson F, Tendal B, Hilden J, Boutron I, et al. Observer bias in randomized clinical trials with measurement scale outcomes: a systematic review of trials with both blinded and nonblinded assessors. Canadian Medical Association Journal 2013;185(4):E201‐11. - PMC - PubMed
HSCIC 2014
-
- Health and Social Care Information Centre. National Child Measurement Programme ‐ England, 2013‐14 school year. www.hscic.gov.uk/ (22 October 2016).
Hsia 2011
Imperatore 2012
James 2010
-
- James WP, Caterson ID, Coutinho W, Finer N, Gaal LF, Maggioni AP, et al. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. New England Journal of Medicine 2010;363(10):905‐17. - PubMed
Kakinami 2014
Kelly 2013
-
- Kelly AS, Barlow SE, Rao G, Inge TH, Hayman LL, Steinberger J, et al. Severe obesity in children and adolescents: identification, associated health risks, and treatment approaches: a scientific statement From the American Heart Association. Circulation 2013;128:1689‐712. - PubMed
Kirkham 2010
Knai 2012
Kolotkin 1997
-
- Kolotkin RL, Head S, Brookhart A. Construct validity of the Impact of Weight on Quality of Life Questionnaire. Obesity Research 1997;5(5):434‐41. - PubMed
Kolotkin 2001
-
- Kolotkin RL, Crosby RD, Williams GR, Hartley GG, Nicol S. The relationship between health‐related quality of life and weight loss. Obesity Research 2001;9(9):564‐71. - PubMed
Kromeyer‐Hausschild 2001
-
- Kromeyer‐Hausschild K, Wabitsch M, Kunze D. Perzentile fur den Body‐mass‐Index fűr das Kindes‐und Jugendalterunter Heranziehung verschiedener deutscher Stichproben. Monatsschr Kinderheilkd 2001;149:807‐18. [DOI: 10.1007/s001120170107] - DOI
Kuczmarski 2000
-
- Kuczmarski RJ, Ogden CL, Grummer‐Strawn LM, Flegal KM, Guo SS, Wei R, et al. CDC growth charts: United States. Advance Data 2000;314:1‐27. - PubMed
Leclercq 2013
-
- Leclercq E, Leeflang MM, Dalen EC, Kremer LC. Validation of search filters for identifying pediatric studies in PubMed. Journal of Pediatrics 2013;162(3):629‐34. - PubMed
Liberati 2009
Lobstein 2004
-
- Lobstein T, Baur L, Uauy R. Obesity in children and young people: a crisis in public health. Obesity Review 2004;5(Suppl 1):4‐104. - PubMed
Loveman 2015
Maahs 2014
-
- Maahs DM, Daniels SR, Ferranti SD, Dichek HL, Flynn J, Goldstein BI, et al. Cardiovascular disease risk factors in youth with diabetes mellitus: a scientific statement from the American Heart Association. Circulation 2014;130(17):1532‐58. - PubMed
Matson 2012
McCreight 2016
McDonagh 2014
McGovern 2008
-
- McGovern L, Johnson JN, Paulo R, Hettinger A, Singhal V, Kamath C, et al. Treatment of pediatric obesity: a systematic review and meta‐analysis of randomized trials. Journal of Clinical Endocrinology and Metabolism 2008;93(12):4600‐5. - PubMed
Meader 2014
Nespoli 2013
-
- Nespoli L, Caprioglio A, Brunetti L, Nosetti L. Obstructive sleep apnea syndrome in childhood. Early Human Development 2013;89(Suppl 3):S33‐7. - PubMed
Ng 2014
NICE 2014
-
- National Institute for Health and Care Excellence. Obesity: identification, assessment and management of overweight and obesity in children, young people and adults. www.nice.org.uk/guidance/cg189/chapter/1‐recommendations#pharmacological... (accessed 5 August 2015). - PubMed
Olds 2011
Padwal 2003
Pandey 2015
-
- Pandey A, Chawla S, Guchhait P. Type‐2 diabetes: current understanding and future perspectives. IUBMB Life 2015;67(7):506‐13. - PubMed
Park 2009
Parsons 1999
-
- Parsons TJ, Power C, Logan S, Summerbell CD. Childhood predictors of adult obesity: a systematic review. International Journal of Obesity 1999;23(Suppl 8):S1‐107. - PubMed
Petkar 2013
Pinhas‐Hamiel 2005
-
- Pinhas‐Hamiel O, Zeitler P. The global spread of type 2 diabetes mellitus in children and adolescents. Journal of Pediatrics 2005;146:693‐700. - PubMed
Power 2014
-
- Power C, Pinto Pereira SM, Law C, Ki M. Obesity and risk factors for cardiovascular disease and type 2 diabetes: investigating the role of physical activity and sedentary behaviour in mid‐life in the 1958 British cohort. Atherosclerosis. 2014;233(2):363‐9. - PubMed
Puhl 2007
-
- Puhl RM, Latner JD. Stigma, obesity, and the health of the nation's children. Psychology Bulletin 2007;133(4):557‐80. - PubMed
Rajput 2014
Ravens‐Sieberer 2001
-
- Ravens‐Sieberer U, Redegeld M, Bullinger M. Quality of life after in‐patient rehabilitation in children with obesity. International Journal of Obesity and Related Metabolic Disorders 2001;25 Suppl 1:S63‐5. - PubMed
Reilly 2003
Reilly 2011
-
- Reilly JJ, Kelly J. Long‐term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: systematic review. International Journal of Obesity 2011;35:891‐8. - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riley 2011
-
- Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta‐analyses. BMJ 2011;342:d549. - PubMed
Rokholm 2010
-
- Rokholm B, Baker JL, Sørenson TIA. The levelling off of the obesity epidemic since the year 1999 ‐ a review of evidence and perspectives. Obesity Reviews 2010;11:835‐46. - PubMed
Rosner 1998
-
- Rosner B, Prineas R, Loggie J, Daniels SR. Percentiles for body mass index in U.S. children 5 to 17 years of age. Journal of Pediatrics 1998;132:211‐22. - PubMed
Sherafat‐Kazemzadeh 2013
Shrewsbury 2008
-
- Shrewsbury V, Wardle J. Socioeconomic status and adiposity in childhood: a systematic review of cross‐sectional studies 1990‐2005. Obesity (Silver Spring) 2008;16(2):275‐84. - PubMed
Singh 2008
-
- Singh AS, Mulder C, Twisk JW, vanMechelen W, Chinapaw MJ. Tracking of childhood overweight into adulthood: a systematic review of the literature. Obesity Reviews 2008;9(5):474‐88. - PubMed
Skinner 2014
Sterne 2011
-
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ 2011;343:d4002. - PubMed
Stunkard 1985
-
- Stunkard AJ, Messick S. The three‐factor eating questionnaire to measure dietary restraint, disinhibition and hunger. Journal of Psychosomatic Research 1985;29:71‐83. - PubMed
Tang‐Peronard 2008
-
- Tang‐Peronard JL, Heitmann BL. Stigmatization of obese children and adolescents, the importance of gender. Obesity Reviews 2008;9:1467‐89. - PubMed
Van Marken Lichtenbelt 1999
-
- Marken Lichtenbelt W, Fogelholm M. Body composition. In: Westerterp‐Plantenga MS, Steffens AB, Tremblay A, eds editor(s). Regulation of Food Intake and Energy Expenditure. Milan: EDRA, 1999:383‐404.
Viner 2010
-
- Viner RM, Hsia Y, Tomsic T, Wong IC. Efficacy and safety of anti‐obesity drugs in children and adolescents: systematic review and meta‐analysis. Obesity Reviews 2010;11(8):593‐602. - PubMed
von Scheven 2006
-
- Scheven E, Gordon CM, Wypij D, Wertz M, Gallagher KT, Bachrach L. Variable deficits of bone mineral despite chronic glucocorticoid therapy in pediatric patients with inflammatory diseases: a Glaser Pediatric Research Network study. Journal of Pediatric Endocrinology & Metabolism 2006;19(6):821‐30. - PMC - PubMed
Wang 2003
-
- Wang J, Thornton JC, Bari S, Williamson B, Gallagher D, Heymsfield SB, et al. Comparisons of waist circumferences measured at 4 sites. American Journal of Clinical Nutrition 2003;77(2):379‐84. - PubMed
Wang 2012
Weiss 2004
-
- Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, et al. Obesity and the metabolic syndrome in children and adolescents. New England Journal of Medicine 2004;350(23):2362‐74. - PubMed
Whitaker 1997
-
- Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. New England Journal of Medicine 1997;337(13):869‐73. [MEDLINE: ] - PubMed
WHO 1995
-
- World Health Organization. The Use and Interpretation of Antropometry. Geneva: WHO 1995.
WHO 2006
-
- World Health Organization. The WHO Child Growth Standards. www.who.int/childgrowth/standards/Technical_report.pdf 2006 (accessed 08/11/2016).
WHO 2015
-
- World Health Organization. Fact sheet on overweight and obesity. www.who.int/mediacentre/factsheets/fs311/en/ (accessed 24 February 2015).
Widhalm 2016
-
- Widhalm HK, Seemann R, Hamboeck M, Mittlboeck M, Neuhold A, Friedrich K, et al. Osteoarthritis in morbidly obese children and adolescents, an age‐matched controlled study. Knee Surgery, Sports Traumatology, Arthroscopy 2016;24(3):644‐52. - PubMed
Wong 2006a
Wood 2008
World Bank 2015
-
- The World Bank. Country and Lending Groups. data.worldbank.org/about/country‐and‐lending‐groups (accessed 5 August 2015).
Yanovski 2014
References to other published versions of this review
Oude Luttikhuis 2009
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

