Estrogen and thyroid cancer is a stem affair: A preliminary study
- PMID: 27899250
- PMCID: PMC5218826
- DOI: 10.1016/j.biopha.2016.11.043
Estrogen and thyroid cancer is a stem affair: A preliminary study
Abstract
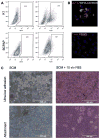
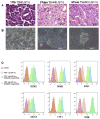
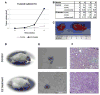
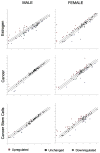
Gender influences Papillary Thyroid Cancer (PTC) with an incidence of 3:1 when comparing women to men with different aggressiveness. This gender discrepancy suggests some role of sex hormones in favoring the malignant progression of thyroid tissue to cancer. Estrogens are known to promote Stem Cell self-renewal and, therefore, may be involved in tumor initiation. The goals of these studies are to investigate the underlying causes of gender differences in PTC by studying the specific role of estrogens on tumor cells and their involvement within the Cancer Stem Cell (CSC) compartment. Exposure to 1nmoll-1 Estradiol for 24h promotes growth and maintenance of PTC Stem Cells, while inducing dose-dependent cellular proliferation and differentiation following Estradiol administration. Whereas mimicking a condition of hormonal imbalance led to an opposite phenotype compared to a continuous treatment. In vivo we find that Estradiol promotes motility and tumorigenicity of CSCs. Estradiol-treated mice inoculated with Thyroid Cancer Stem Cell-enriched cells developed larger tumor masses than control mice. Furthermore, Estradiol-pretreated Cancer Stem cells migrated to distant organs, while untreated cells remained circumscribed. We also find that the biological response elicited by estrogens on Papillary Thyroid Cancer in women differed from men in pathways mediated. This could explain the gender imbalance in tumor incidence and development and could be useful to develop gender specific treatment of (PTC).
Keywords: Cancer signaling; Cancer stem cells; Estrogen; Gender medicine; Thyroid cancer.
Copyright © 2016 Elsevier Masson SAS. All rights reserved.
Conflict of interest statement
Declaration of interest The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Figures



References
-
- Ahmed RA, Aboelnaga EM. Thyroid cancer in Egypt: histopathological criteria, correlation with survival and oestrogen receptor protein expression. Pathol Oncol Res. 2015;21:793–802. - PubMed
-
- Antico Arciuch VG, Di Cristofano A. Estrogen signaling and thyrocyte proliferation. In: Ward DL, editor. Thyroid and Parathyroid Diseases-New insights into Some Old and Some New Issues. 2012.
-
- Caini S, Gibelli B, Palli D, Saieva C, Ruscica M, Gandini S. Menstrual and reproductive history and use of exogenous sex hormones and risk of thyroid cancer among women: a meta-analysis of prospective studies. Cancer Causes Control. 2015;26:511–518. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

