Combining transcription factor binding affinities with open-chromatin data for accurate gene expression prediction
- PMID: 27899623
- PMCID: PMC5224477
- DOI: 10.1093/nar/gkw1061
Combining transcription factor binding affinities with open-chromatin data for accurate gene expression prediction
Abstract
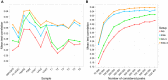
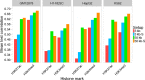
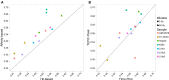
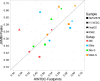
The binding and contribution of transcription factors (TF) to cell specific gene expression is often deduced from open-chromatin measurements to avoid costly TF ChIP-seq assays. Thus, it is important to develop computational methods for accurate TF binding prediction in open-chromatin regions (OCRs). Here, we report a novel segmentation-based method, TEPIC, to predict TF binding by combining sets of OCRs with position weight matrices. TEPIC can be applied to various open-chromatin data, e.g. DNaseI-seq and NOMe-seq. Additionally, Histone-Marks (HMs) can be used to identify candidate TF binding sites. TEPIC computes TF affinities and uses open-chromatin/HM signal intensity as quantitative measures of TF binding strength. Using machine learning, we find low affinity binding sites to improve our ability to explain gene expression variability compared to the standard presence/absence classification of binding sites. Further, we show that both footprints and peaks capture essential TF binding events and lead to a good prediction performance. In our application, gene-based scores computed by TEPIC with one open-chromatin assay nearly reach the quality of several TF ChIP-seq data sets. Finally, these scores correctly predict known transcriptional regulators as illustrated by the application to novel DNaseI-seq and NOMe-seq data for primary human hepatocytes and CD4+ T-cells, respectively.
© The Author(s) 2016. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures



References
-
- Vaquerizas J.M., Kummerfeld S.K., Teichmann S.A., Luscombe N.M. A census of human transcription factors: function, expression and evolution. Nat. Rev. Genet. 2009;10:252–263. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

