Structures of human SRP72 complexes provide insights into SRP RNA remodeling and ribosome interaction
- PMID: 27899666
- PMCID: PMC5224484
- DOI: 10.1093/nar/gkw1124
Structures of human SRP72 complexes provide insights into SRP RNA remodeling and ribosome interaction
Abstract
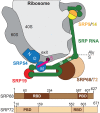
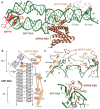
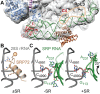
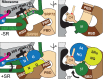
Co-translational protein targeting and membrane protein insertion is a fundamental process and depends on the signal recognition particle (SRP). In mammals, SRP is composed of the SRP RNA crucial for SRP assembly and function and six proteins. The two largest proteins SRP68 and SRP72 form a heterodimer and bind to a regulatory site of the SRP RNA. Despite their essential roles in the SRP pathway, structural information has been available only for the SRP68 RNA-binding domain (RBD). Here we present the crystal structures of the SRP68 protein-binding domain (PBD) in complex with SRP72-PBD and of the SRP72-RBD bound to the SRP S domain (SRP RNA, SRP19 and SRP68) detailing all interactions of SRP72 within SRP. The SRP72-PBD is a tetratricopeptide repeat, which binds an extended linear motif of SRP68 with high affinity. The SRP72-RBD is a flexible peptide crawling along the 5e- and 5f-loops of SRP RNA. A conserved tryptophan inserts into the 5e-loop forming a novel type of RNA kink-turn stabilized by a potassium ion, which we define as K+-turn. In addition, SRP72-RBD remodels the 5f-loop involved in ribosome binding and visualizes SRP RNA plasticity. Docking of the S domain structure into cryo-electron microscopy density maps reveals multiple contact sites between SRP68/72 and the ribosome, and explains the role of SRP72 in the SRP pathway.
© The Author(s) 2016. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures



References
-
- Nyathi Y., Wilkinson B.M., Pool M.R. Co-translational targeting and translocation of proteins to the endoplasmic reticulum. Biochim. Biophys. Acta Mol. Cell Res. 2013;1833:2392–2402. - PubMed
-
- Voorhees R.M., Hegde R.S. Toward a structural understanding of co-translational protein translocation. Curr. Opin. Cell Biol. 2016;41:91–99. - PubMed
-
- Egea P.F., Shan S.-O., Napetschnig J., Savage D.F., Walter P., Stroud R.M. Substrate twinning activates the signal recognition particle and its receptor. Nature. 2004;427:215–221. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

