Cellular and Molecular Responses to Mechanical Expansion of Tissue
- PMID: 27899897
- PMCID: PMC5111402
- DOI: 10.3389/fphys.2016.00540
Cellular and Molecular Responses to Mechanical Expansion of Tissue
Abstract
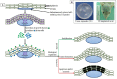
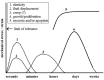
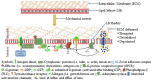
The increased use of tissue expander in the past decades and its potential market values in near future give enough reasons to sum up the consequences of tissue expansion. Furthermore, the patients have the right to know underlying mechanisms of adaptation of inserted biomimetic, its bioinspired materials and probable complications. The mechanical strains during tissue expansion are related to several biological phenomena. Tissue remodeling during the expansion is highly regulated and depends on the signal transduction. Any alteration may lead to tumor formation, necrosis and/or apoptosis. In this review, stretch induced cell proliferation, apoptosis, the roles of growth factors, stretch induced ion channels, and roles of second messengers are organized. It is expected that readers from any background can understand and make a decision about tissue expansion.
Keywords: apoptosis; focal adhesion complex; growth factors; ion channels; secondary messengers; tissue expansion.
Figures

Similar articles
-
Mechanical Stretch Induced Skin Regeneration: Molecular and Cellular Mechanism in Skin Soft Tissue Expansion.Int J Mol Sci. 2022 Aug 25;23(17):9622. doi: 10.3390/ijms23179622. Int J Mol Sci. 2022. PMID: 36077018 Free PMC article. Review.
-
Mechanical tension as a driver of connective tissue growth in vitro.Med Hypotheses. 2014 Jul;83(1):111-5. doi: 10.1016/j.mehy.2014.03.031. Epub 2014 Apr 5. Med Hypotheses. 2014. PMID: 24755458
-
Tuning outer segment Ca2+ homeostasis to phototransduction in rods and cones.Adv Exp Med Biol. 2002;514:179-203. doi: 10.1007/978-1-4615-0121-3_11. Adv Exp Med Biol. 2002. PMID: 12596922 Review.
-
Autologous Stem Cell Transplantation Promotes Mechanical Stretch Induced Skin Regeneration: A Randomized Phase I/II Clinical Trial.EBioMedicine. 2016 Nov;13:356-364. doi: 10.1016/j.ebiom.2016.09.031. Epub 2016 Oct 1. EBioMedicine. 2016. PMID: 27876353 Free PMC article. Clinical Trial.
-
[Cell responses to mechanical stresses: mechano-sensors and messengers].Clin Calcium. 2008 Sep;18(9):1295-303. Clin Calcium. 2008. PMID: 18758035 Review. Japanese.
Cited by
-
The Use of Self-Inflating Hygroscopic Tissue Expanders to Facilitate Osteosarcoma Removal in a Massasauga Rattlesnake (Sistrurus catenatus).Case Rep Vet Med. 2020 Jul 28;2020:8813911. doi: 10.1155/2020/8813911. eCollection 2020. Case Rep Vet Med. 2020. PMID: 32774984 Free PMC article.
-
Dynamics of cutaneous atmospheric oxygen uptake in response to mechanical stretch revealed by optical fiber microsensor.Exp Dermatol. 2023 Dec;32(12):2112-2120. doi: 10.1111/exd.14957. Epub 2023 Oct 19. Exp Dermatol. 2023. PMID: 37859506 Free PMC article.
-
The Roles of WNT Signaling Pathways in Skin Development and Mechanical-Stretch-Induced Skin Regeneration.Biomolecules. 2023 Nov 24;13(12):1702. doi: 10.3390/biom13121702. Biomolecules. 2023. PMID: 38136575 Free PMC article. Review.
-
S100 calcium-binding protein A9 promotes skin regeneration through toll-like receptor 4 during tissue expansion.Burns Trauma. 2023 Oct 31;11:tkad030. doi: 10.1093/burnst/tkad030. eCollection 2023. Burns Trauma. 2023. PMID: 37936894 Free PMC article.
-
Transcriptome Profiling Reveals Important Transcription Factors and Biological Processes in Skin Regeneration Mediated by Mechanical Stretch.Front Genet. 2021 Sep 29;12:757350. doi: 10.3389/fgene.2021.757350. eCollection 2021. Front Genet. 2021. PMID: 34659370 Free PMC article.
References
-
- Amendt C., Mann A., Schirmacher P., Blessing M. (2002). Resistance of keratinocytes to TGFβ-mediated growth restriction and apoptosis induction accelerates re-epithelialization in skin wounds. J. Cell Sci. 115, 2189–2198. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

