Next-generation sequencing in NSCLC and melanoma patients: a cost and budget impact analysis
- PMID: 27899957
- PMCID: PMC5102690
- DOI: 10.3332/ecancer.2016.684
Next-generation sequencing in NSCLC and melanoma patients: a cost and budget impact analysis
Abstract
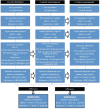
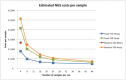
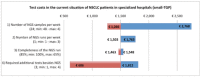
Next-generation sequencing (NGS) has reached the molecular diagnostic laboratories. Although the NGS technology aims to improve the effectiveness of therapies by selecting the most promising therapy, concerns are that NGS testing is expensive and that the 'benefits' are not yet in relation to these costs. In this study, we give an estimation of the costs and an institutional and national budget impact of various types of NGS tests in non-small-cell lung cancer (NSCLC) and melanoma patients within The Netherlands. First, an activity-based costing (ABC) analysis has been conducted on the costs of two examples of NGS panels (small- and medium-targeted gene panel (TGP)) based on data of The Netherlands Cancer Institute (NKI). Second, we performed a budget impact analysis (BIA) to estimate the current (2015) and future (2020) budget impact of NGS on molecular diagnostics for NSCLC and melanoma patients in The Netherlands. Literature, expert opinions, and a data set of patients within the NKI (n = 172) have been included in the BIA. Based on our analysis, we expect that the NGS test cost concerns will be limited. In the current situation, NGS can indeed result in higher diagnostic test costs, which is mainly related to required additional tests besides the small TGP. However, in the future, we expect that the use of whole-genome sequencing (WGS) will increase, for which it is expected that additional tests can be (partly) avoided. Although the current clinical benefits are expected to be limited, the research potentials of NGS are already an important advantage.
Keywords: NSCLC; Next-generation sequencing; budget impact; melanoma; personalised medicine; targeted therapy; test costs.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
