Therapeutic targeting of liver cancer with a recombinant DNA vaccine containing the hemagglutinin-neuraminidase gene of Newcastle disease virus via apoptotic-dependent pathways
- PMID: 27900002
- PMCID: PMC5103948
- DOI: 10.3892/ol.2016.5114
Therapeutic targeting of liver cancer with a recombinant DNA vaccine containing the hemagglutinin-neuraminidase gene of Newcastle disease virus via apoptotic-dependent pathways
Abstract
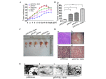
A total of ~38.6 million mortalities occur due to liver cancer annually, worldwide. Although a variety of therapeutic methods are available, the efficacy of treatment at present is extremely limited due to an increased risk of malignancy and inherently poor prognosis of liver cancer. Gene therapy is considered a promising option, and has shown notable potential for the comprehensive therapy of liver cancer, in keeping with advances that have been made in the development of cancer molecular biology. The present study aimed to investigate the synergistic effects of the abilities of the hemagglutinin neuraminidase protein of Newcastle disease virus (NDV), the pro-apoptotic factor apoptin from chicken anaemia virus, and the interferon-γ inducer interleukin-18 (IL-18) in antagonizing liver cancer. Therefore, a recombinant DNA plasmid expressing the three exogenous genes, VP3, IL-18 and hemagglutinin neuraminidase (HN), was constructed. Flow cytometry, acridine orange/ethidium bromide staining and analysis of caspase-3 activity were performed in H22 cell lines transfected with the recombinant DNA plasmid. In addition, 6-week-old C57BL/6 mice were used to establish a H22 hepatoma-bearing mouse model. Mice tumor tissue was analyzed by immunohistochemistry and scanning electron microscopy. The results of the present study revealed that the recombinant DNA vaccine containing the VP3, IL-18 and HN genes inhibited cell proliferation and induced autophagy via the mitochondrial pathway in vivo and in vitro.
Keywords: Newcastle disease virus; apoptosis; gene therapy; hemagglutinin neuraminidase gene; hepatocellular carcinoma; recombinant plasmid.
Figures


References
-
- Baliaka A, Zarogoulidis P, Domvri K, Hohenforst-Schmidt W, Sakkas A, Huang H, Le Pivert P, Koliakos G, Koliakou E, Kouzi-Koliakos K, et al. Intratumoral gene therapy versus intravenous gene therapy for distant metastasis control with 2-diethylaminoethyl-dextran methyl methacrylate copolymer non-viral vector-p53. Gene Ther. 2014;21:158–167. doi: 10.1038/gt.2013.68. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
